FDA Strengthens Infant Formula Recall Oversight After Botulism Case

The U.S. Food and Drug Administration has announced new actions aimed at improving recall effectiveness following an investigation into an infant botulism illness. The agency said the case exposed gaps in how recalls are communicated and verified, prompting a renewed focus on accountability across the food supply chain. While infant botulism is rare, the FDA
... Read moreHow Combining Ingredients For A GRAS Conclusion Affects Product Safety

Introduction When working toward a GRAS conclusion for an ingredient, a common question is whether it is possible to combine multiple ingredients and support one clear safety position. Many teams want to understand if individually safe ingredients will still meet expectations once they are used together in the same formulation. The FDA expects you to
... Read moreNNHPD Update: New Tools for Monograph Combinations and Interchangeable Terms

NNHPD has introduced two new regulatory tools designed to simplify the natural health product submission process and give you more flexibility when preparing labels. Moreover, these updates were created to improve clarity in submission pathways and support modern, consistent labelling practices across the industry. If you work with NHPs or non-prescription drugs, you will want to understand these
... Read moreA Guide to Toxicological Studies for GRAS Conclusion

Building your safety narrative for a GRAS notice begins with toxicological studies for GRAS conclusion, and this foundation is one of the most important parts of proving that a food ingredient is safe for its intended use. As you prepare a GRAS notice or work toward a self-affirmed GRAS position, you also need solid scientific evidence that
... Read moreUnderstanding the Differences When Importing Human and Animal Food into Canada

When it comes to importing human food and animal food into Canada, many businesses assume the process is similar. In reality, while both categories are regulated to protect consumers and animals, the requirements, permits, and oversight agencies differ significantly. Understanding these distinctions is crucial if you plan to expand into the Canadian market. In this
... Read moreGetting an NPN for Sports Supplements: Health Canada Step-By-Step Guide

Getting an NPN for sports supplements is essential for businesses looking to market their products legally in Canada. The Natural Product Number (NPN) is a unique identifier issued by Health Canada that confirms a product meets safety, efficacy, and quality standards. If you are involved in the sports nutrition industry, understanding how to obtain an
... Read moreTime to Renew: FDA Drug Establishment and Listing Deadline Is Coming Up

If your company manufactures, repacks, relabels, or imports drugs for distribution in the U.S., it’s time to prepare for the FDA drug establishment renewal season. Every year, between October 1 and December 31, the Food and Drug Administration (FDA) requires all registered drug establishments to renew their registration and certify their product listings. Missing this
... Read moreUnderstanding CETA and How It Helps You Sell Food Supplements in Canada Tariff Free

When exporting food supplements to Canada, understanding trade agreements can make a huge difference in your profitability. The Comprehensive Economic and Trade Agreement (CETA) between Canada and the European Union opens valuable opportunities for EU businesses and helps simplify trade procedures. Through the CETA trade agreement, food supplement exporters can eliminate tariffs, reach Canadian consumers
... Read moreExpert Panel in GRAS Determinations: Why It Matters for Ingredient Safety

When it comes to ingredient safety, an expert panel plays one of the most critical roles in the GRAS (Generally Recognized as Safe) process. These independent experts bring credibility and scientific validation to your safety determination, especially when pursuing the self-affirmed GRAS route. In this guide, you will learn what an expert panel does, when
... Read moreYour Guide to Achieving GRAS Approval for Enzymes

Getting GRAS status for an enzyme is one of the most important steps to ensure your ingredient is recognized as safe for its intended use. GRAS, which stands for Generally Recognized as Safe, is the U.S. Food and Drug Administration’s (FDA) framework that allows companies to confirm ingredient safety without going through a full premarket
... Read moreClinical Studies for EFSA Novel Food Applications: How to Ensure Success

Introduction: Why EFSA Novel Food Clinical Studies Matter Bringing a novel food to the European market requires more than innovation. It requires scientific proof of safety and efficacy that demonstrates how your product interacts with the human body and benefits consumers. If you’re developing a new ingredient, supplement, or functional food, understanding EFSA’s expectations is
... Read moreYour Step-by-Step Guide to the EFSA Novel Food Application Process

If you’re planning to introduce an innovative ingredient or food product in the European Union, understanding how to submit an EFSA novel food application is essential. The European Food Safety Authority (EFSA) ensures that any food not consumed in the EU before May 15, 1997, is scientifically proven to be safe before it reaches the
... Read moreFDA Declares Nicotinamide Mononucleotide Lawful in Dietary Supplements

FDA confirms NMN is lawful in dietary supplements after industry pressure. Learn what this means for your business and compliance strategy.
... Read morePreparing Instructions for Use for FDA Submission

Learn how to prepare Instructions for Use for FDA submission for medical devices. Stay compliant with FDA requirements and international standards.
... Read moreFDA Self-Affirmed GRAS Pathway May Soon End

FDA proposes ending the self-affirmed GRAS pathway. Learn what this means for your business and how to stay compliant.
... Read moreTop SR&ED Eligibility Myths and What Actually Qualifies

When it comes to SR&ED eligibility, many businesses assume their projects do not qualify. This misconception often leads to missed opportunities for valuable tax credits and refunds. The Scientific Research and Experimental Development (SR&ED) program was created to support innovation, but a lack of clarity has left many companies unsure of what qualifies. Understanding the
... Read moreBest Practices for Achieving GRAS Status for Manufacturers

Reaching GRAS status is an important step if you want your Generally Recognized as Safe ingredient accepted for use in the U.S. food and beverage market. It proves to regulators, consumers, and industry partners that your ingredient is safe and backed by science. However, the process can be complex. From study design to FDA expectations,
... Read moreThe 7 Key Sections of a GRAS Dossier Every Business Should Understand

Preparing a GRAS dossier is one of the most important steps when seeking FDA recognition for a new substance in food. This dossier demonstrates that the ingredient is safe for its intended use and provides the scientific evidence needed to achieve Generally Recognized As Safe (GRAS) status. Each of the seven sections in a dossier
... Read moreFDA Launches Real Time Adverse Event Reporting Dashboard for Cosmetic Products

The FDA adverse event reporting dashboard for cosmetic products is now live, offering businesses and consumers real time access to safety data. This launch marks an important milestone under the Modernization of Cosmetics Regulation Act (MoCRA), giving more transparency into how adverse events are tracked and reported. For companies in the cosmetics sector, this dashboard
... Read moreHealth Canada Releases Version 4.0 of the GMP Guide for Natural Health Products
On September 4, 2025, Health Canada released Version 4.0 of the Good Manufacturing Practices (GMP) Guide for Natural Health Products (NHPs). This updated GMP guide takes effect on March 4, 2026. Until then, Version 3 remains in force and will continue to be referenced by inspectors. If your business manufactures, imports, packages, or distributes NHPs,
... Read moreHealth Canada to Reduce Red Tape: What It Means for Your Business

Businesses in Canada have long raised concerns about red tape in Health Canada regulations, from outdated rules to lengthy approval processes and unclear requirements. These challenges can slow down innovation, increase costs, and create unnecessary administrative burden. To address these issues, Health Canada and the Public Health Agency of Canada (PHAC) released a detailed report
... Read moreDe Minimis Rule Ending: What Businesses Must Know

The de minimis rule has long been a key part of U.S. customs policy. It allowed companies to import low-value shipments under $800 without paying duties or submitting full customs paperwork. For years, this policy helped streamline e-commerce and cross-border trade. But now, the U.S. government has announced the end of the de minimis rule,
... Read moreWhat You Need to Know About GMP Training

What is GMP training, and why does it matter? If your company makes, packages, labels, or imports health products, this training is one of the first steps to compliance. Good Manufacturing Practices (GMP) provide the essential framework for maintaining product safety, quality, and consistency across all manufacturing operations. When teams lack this foundational knowledge, they
... Read moreWhat the New FDA PreCheck Program Means for Drug Manufacturers

FDA PreCheck program aims to boost U.S. drug manufacturing. Learn how it could impact domestic pharmaceutical facilities and compliance steps.
... Read moreFDA Proposes Compliance Extension And Releases New Tools For Food Traceability

The FDA food traceability rule is once again in the spotlight. On August 6, 2025, the U.S. Food and Drug Administration (FDA) proposed extending the compliance date for the rule and released new FAQs and other resources to help businesses meet the requirements. If your organization handles foods on the FDA’s Food Traceability List, this
... Read moreHow to Register Your Private Label Medical Device in Canada

Learn how to register a private label medical device with Health Canada. Follow this step-by-step guide to ensure regulatory compliance.
... Read moreFDA Chemical Screening: What The New EDT Tool Means For Food Safety

FDA’s new EDT tool helps screen chemicals in food for toxicity. Learn how it supports safer food and faster evaluations.
... Read moreSenators Propose Bill To Eliminate Self-affirmed GRAS Loophole

A new bill introduced in the U.S. Senate could significantly alter how food ingredient safety is assessed, especially when it comes to self-affirmed GRAS determinations. The proposed changes aim to address long-standing concerns about transparency and potential risks associated with allowing companies to determine the safety of ingredients without regulatory oversight. On July 17, 2025,
... Read moreHow the SAFE Sunscreen Act Could Change FDA Compliance

Sunscreen compliance in the U.S. may be on the edge of its most significant update in over two decades. On June 3, 2025, lawmakers introduced the SAFE Sunscreen Standards Act (H.R.3686), a bipartisan bill that aims to modernize how the FDA reviews and approves sunscreen active ingredients. This matters because no new sunscreen ingredients have
... Read moreAre you meeting Health Canada’s site license renewal requirements?

Over the past few weeks, many natural health product (NHP) companies have been caught off guard by an increase in site license suspensions or renewal refusals. Health Canada is reinforcing its compliance expectations and taking action where gaps are found. If your site license renewal is coming up, this is the time to double-check your
... Read moreWhat Is Changing Under CFIA’s Feed Regulatory Modernization?

Why is CFIA Updating its Feed Regulations? The Canadian Food Inspection Agency (CFIA) is in the process of modernizing its feed regulations to better protect animal health, food safety, and the environment. If you’re a feed manufacturer, importer, or distributor, the CFIA feed regulatory modernization will impact your operations and compliance requirements directly. The new
... Read moreHealth Canada issues interim report on sodium targets for processed food

Introduction Sodium reduction in processed foods is no longer optional. It is a critical step for industry players to safeguard consumer health and meet Health Canada’s 2025 targets. Too much sodium raises blood pressure and increases the risk of heart disease and stroke, which are among the leading causes of death in Canada. In December
... Read moreComparing FDA 510(k) and PMA Pathways for Medical Devices

When preparing to launch a medical device in the U.S., one of the first and most important decisions you’ll make is choosing the correct regulatory pathway. For many manufacturers, that means understanding the FDA 510(k) pre-submission process and how it compares to the Premarket Approval (PMA) route. These two regulatory pathways represent the most common
... Read moreBoosting Business Operations Through GMP Training

If you work in the life sciences industry, you’ve likely heard of GMP, Good Manufacturing Practice. It’s a fundamental part of producing safe, effective, and high-quality products. But how confident are you that your team fully understands what’s expected, and more importantly, why it matters? This blog breaks down what GMP compliance really involves and
... Read moreThe Role of Audits in Meeting Health Canada’s Compliance Standards

When it comes to audits related to Health Canada requirements, staying compliant is about more than checking off a list. It helps you maintain your licence, protect your brand, and stay competitive in a regulated market. If you hold a Drug Establishment Licence (DEL), Medical Device Establishment Licence (MDEL), or a Natural Health Product (NHP)
... Read moreHow to Comply with the FDA’s NDI Notification Process

Learn how to meet FDA’s new dietary ingredient notification requirements for dietary supplements with new educational tools.
... Read morePet Supplements or Veterinary Health Products? Understanding the Difference

If you are planning to market animal health products, the question of pet supplements or veterinary health products will come up fast. This difference matters, especially if you’re selling in both the United States and Canada. What qualifies as a supplement in the U.S. might be considered a drug or a Veterinary Health Product
... Read moreClass II NHP submissions: What Health Canada’s New Workload Rules Mean For You

Starting June 9, 2025, Class II NHP submissions will no longer be reviewed just based on the date you filed them. Health Canada is updating how it handles licensing applications by applying a new workload management approach, prioritizing submissions based on intent to sell or manufacture in Canada.
... Read moreA Guide to Securing an NPN for Hydration Products in Canada

Learn how to get an NPN for hydration products in Canada and ensure full Health Canada compliance.
... Read moreGRAS for Food vs. Feed: Understanding the Key Differences

When introducing a new substance into the market, one of the first questions you need to answer is whether it’s intended for human food or animal feed. This decision significantly impacts how the substance must be evaluated and documented under the GRAS (Generally Recognized as Safe) process. The regulatory pathway for GRAS differs depending on
... Read moreTraditional Cosmetics vs. Cosmeceuticals: What’s the Difference?

Understanding the distinction between traditional cosmetics vs. cosmeceuticals is essential if you’re manufacturing or marketing skincare or beauty products in North America. While both types of products may appear similar on store shelves, they follow different regulatory pathways, and that has a direct impact on your compliance obligations. Whether you’re selling moisturizers, anti-aging serums, or
... Read moreA Guide to the SFCR Importer of Record Role

Learn what an SFCR importer of record is and how CARM changes affect food importers in Canada. Stay compliant with expert support.
... Read moreStruggling With GRAS Compliance? Here’s What to Do

Learn what to do if you’re struggling with the GRAS compliance process. Follow these tips to avoid delays and meet FDA standards.
... Read moreFDA Approves Natural Food Colors for Safer Use

The FDA has officially approved three new food color additives derived from natural sources. These offer a safer alternative to petroleum-based dyes in the U.S. food supply. The approval of these natural food colors marks a big step toward cleaner labeling and healthier food options. Moreover, this update supports the U.S. Department of Health and
... Read moreFDA Unannounced Inspections: What Foreign Manufacturers Need To Know

FDA unannounced inspections are no longer limited to U.S. facilities. The U.S. Food and Drug Administration (FDA) recently announced an expansion of its unannounced inspection program to now include foreign manufacturing sites. This change will directly impact companies exporting pharmaceuticals and other regulated products into the U.S. If you rely on international partners or operate
... Read moreHealth Canada electronic filing updates: Key changes in 2025

If your team handles drug product submissions in Canada, there’s a recent update from Health Canada you might want to take a closer look at. As of April 1, 2025, new electronic filing rules are now in place for both eCTD and non-eCTD formats. These updates aim to make the submission process more streamlined
... Read moreNavigating FDA and Health Canada Rules for Eczema Products

The eczema product market continues to grow as consumer demand increases, but that doesn’t mean you can jump in without a plan. FDA and Health Canada rules for eczema products vary widely depending on the formulation and claims. Many companies risk product delays or enforcement action by misclassifying their product or using unapproved claims. If
... Read moreComparing Cosmetic Compliance Standards: FDA and Health Canada
How FDA and Health Canada Regulate Cosmetics Differently Thinking about launching your cosmetic line in North America? Whether you’re a seasoned brand or just getting started, understanding FDA cosmetic compliance vs Health Canada requirements is a must. Each country has its own set of rules, and missing even one detail can cause delays, product holds,
... Read moreHealth Canada’s April 2025 Updates on the Medical Device Shortage List

Medical device shortage reporting requirements have recently changed, and it is essential for manufacturers and importers to stay informed.
... Read morePreparing a Successful Food Additive Submission in Canada

Learn how to prepare a food additive submission for Health Canada to ensure compliance and avoid costly delays.
... Read moreTips to Keep Cosmetics Compliant and Listed on Amazon Canada

Selling cosmetics on Amazon Canada can open up a world of opportunity, but it comes with strict compliance expectations. Recently, many sellers have faced Amazon Canada cosmetic delisting due to missing or incomplete regulatory documentation. This issue not only disrupts sales but can damage your brand’s reputation and standing with Amazon. To prevent these setbacks,
... Read moreFDA announces ESG NextGen launch for industry submissions

FDA ESG NextGen is now officially live. If your company needs to submit regulatory documents to the FDA, you’ll now use this updated system instead of the retired WebTrader platform. The launch marks a major infrastructure upgrade. With improved file handling, automated submission tracking, and strengthened cybersecurity, ESG NextGen is designed to reduce submission errors,
... Read moreEFSA Opens 2025 Call for Novel Food Advice to SMEs

Are you a small or medium-sized business (SME) planning to launch a novel food in the European Union? If so, this is your chance to receive free EFSA novel food advice before submitting your application. The European Food Safety Authority (EFSA) has launched its second call for expressions of interest, specifically targeting SMEs preparing for
... Read moreWhat’s the Difference Between Functional Foods and NHPs?

Learn the difference between functional foods and Natural Health Products in Canada, and how to classify your product correctly.
... Read moreThe Role of Natural Product Numbers (NPNs) in Canada

What is NPN? Learn how to get approval for your natural health product and stay compliant with Canadian regulations.
... Read moreFood and Supplement Labeling: Canada vs US Requirements

Understand food and supplement labeling differences between Canada and the US to stay compliant and avoid costly delays.
... Read moreFDA Releases Cosmetic Facility & Product Registration Summary

On March 13, 2025, the U.S. Food and Drug Administration (FDA) released its first public summary data on the mandatory registration of cosmetic product facilities and the listing of cosmetic products, as required under the Modernization of Cosmetics Regulation Act of 2022 (MoCRA). If you’re a manufacturer, processor, or brand owner distributing cosmetics in the
... Read moreFDA to Extend Food Traceability Rule: What You Need to Know

The FDA plans to extend the Food Traceability Rule compliance date by 30 months. Find out how this impacts your compliance strategy.
... Read moreHealth Canada Announces Amendments to Cannabis Regulations

As of March 12, 2025 Health Canada has implemented changes to the Cannabis Act and its Regulations. These changes may impact licensed operations as well as current applicants. While some of these changes apply immediately, others have transition periods to give businesses time to adjust. Understanding these updates now can help you stay compliant, cut
... Read moreHealth Canada Proposes Changes to Caffeine as a Supplemental Ingredient

Changes are on the horizon for caffeine as a supplemental ingredient, and they could directly impact your business. Whether you manufacture energy drinks, snack bars, or functional beverages, these updates will determine how you formulate and market your products moving forward. At the same time, Health Canada is tightening regulations to ensure consumers stay informed
... Read moreSelf-Affirmed GRAS May End – What It Means for You

The self-affirmed GRAS pathway may soon be eliminated. On March 10, 2025, HHS Secretary Robert F. Kennedy Jr. directed the FDA to explore rulemaking to remove the option for companies to self-affirm that food ingredients are safe. If implemented, this shift will require all GRAS determinations to undergo FDA review, significantly altering the regulatory landscape
... Read moreFDA’s New AI Rules for Medical Devices: What’s Changing?

The FDA just updated its AI rules for medical devices. Learn what’s changing, how it will impact your business, and stay ahead of compliance updates.
... Read moreNHP Labelling: Ministerial Exemption Order Announced

Health Canada announces a Ministerial Exemption Order for NHP labelling regulations, delaying new compliance requirements until June 21, 2028.
... Read moreIs Your Cosmetic Ready for Canada’s New Allergen Rules?

Health Canada’s fragrance allergen rules take effect April 12, 2026. Ensure your cosmetic products comply with new labelling regulations.
... Read moreHealth Canada’s New Front-of-Package Label Rules Explained

Introduction Health Canada’s new front-of-package label rules are set to take effect on January 1, 2026, marking a significant shift in how food products must be labelled. These regulations are designed to help consumers make informed choices by clearly identifying products high in saturated fat, sugars, or sodium. If you’re a food manufacturer, retailer, or
... Read moreHow to Make ‘Made in Canada’ Claims Amid Trade Tensions

Understanding ‘Made in Canada’ Claims With trade tensions rising and new tariffs affecting cross-border business, Canadian brands must ensure they accurately label their products. Claiming a product is “Made in Canada” can boost consumer trust and appeal, but businesses must follow strict regulations to avoid misleading advertising. Recent changes and increased scrutiny from the Competition
... Read moreNavigating the TGA Approval Process for New Substances

Discover the key steps to obtaining TGA approval for new substances in listed medicines. Ensure compliance and streamline your application.
... Read moreA Practical Compliance Guide to Shilajit and FDA Rules

Navigating the FDA compliance process for shilajit can be challenging, especially if you’re new to the regulatory landscape. As shilajit gains popularity in the U.S. for its health benefits, it’s crucial to ensure your product meets FDA requirements before introducing it to the market. Understanding these steps from the beginning can help you streamline the
... Read moreUnderstanding Health Canada’s Approval on Grape Seed Extract in Foods

Health Canada has made an important decision regarding grape seed extract, now approved for use as a supplemental ingredient in foods. This modification marks a major development for businesses operating in the health and nutrition sector. Keep reading to learn how this change can affect your products and compliance strategies. Health Canada’s Decision on Grape
... Read moreWhy Are FDA Food Contact Notifications No Longer Effective?

Have you heard about the recent updates to FDA Food Contact Notifications (FCN)? The FDA has determined that several FCN are no longer effective due to changes in manufacturing and regulatory standards. These updates directly affect businesses that rely on food contact substances (FCS). Furthermore, they include strict compliance deadlines that organizations must meet. Understanding
... Read moreWhat Are FDA Medical Device Classes?
Understanding the FDA classification system for medical devices is key to getting your product to market safely and efficiently. Knowing how the FDA medical devices are categorized can help you avoid setbacks and ensure compliance, whether you’re dealing with a simple tool or a life-saving implant. But how exactly does the FDA determine a device’s
... Read moreHealth Canada Fees 2025 Guide

Understand Health Canada’s 2025 fees for NHPs, drugs, medical devices, and cannabis. Stay compliant with Quality Smart Solutions.
... Read moreHealth Canada’s New Nicotine Rules: What’s Changing in 2024

If you’re in the business of nicotine products like pouches, gums, patches, sprays, or lozenges (collectively known as nicotine replacement therapies, or NRTs), you need to know about Health Canada’s new nicotine rules. Major regulatory changes are now in place to ensure NRT products are marketed responsibly, labeled clearly, and sold in ways that prioritize
... Read moreHow to Prepare for an FDA 510(k) Pre-Submission

Are you trying to make sense of the FDA’s 510(k) pre-submission process? You’re not alone. Many companies, especially those new to the regulatory landscape, find themselves wondering where to start, what’s required, and how to get FDA approval for their medical devices. Without a clear plan, it’s easy to waste time and money on unnecessary
... Read moreHow to Ensure Compliance with Canada’s Infant Formula Registration

Are you looking to bring your infant formula to the Canadian market? Successfully navigating Canada infant formula registration is essential to ensure compliance with Health Canada’s strict regulations. Canada enforces some of the world’s strictest regulations to ensure that infant formulas meet the highest standards for safety, quality, and nutrition. Successfully navigating this process requires
... Read moreHealth Canada Opens 75-Day Consultation on Batch 4b – Share Your Input

Health Canada Opens 75-Day Consultation on Batch 4b – Share Your Input The Natural and Non-Prescription Health Products Directorate (NNHPD) invites industry stakeholders to provide feedback on Batch 4b, with consultation open for 75 days. We encourage you to support any suggestions for monograph revisions with published evidence or clear rationale. What’s Included in Batch
... Read moreHow to Master FDA Audits for Your Dietary Supplement Facility: A Complete Preparation Guide

Introduction Preparing for an FDA audit can be challenging, especially for dietary supplement manufacturers who must meet stringent Good Manufacturing Practices (GMP) requirements. The FDA regularly inspects dietary supplement facilities to ensure manufacturing, labeling, and safety regulations compliance. But how can you ensure you’re ready? Failing to meet FDA standards can lead to severe consequences,
... Read moreHealth Canada’s NDS, SNDS, and ANDS Pathways: Which One Is Right for You?
If you’re thinking about bringing a pharmaceutical product to the Canadian market, getting familiar with drug submission pathways is a crucial first step. These regulatory routes can seem a bit confusing at first, but understanding them will save you time, money, and potential setbacks. Whether you’re introducing a new drug, modifying an existing one, or
... Read moreHealth Canada’s Drug vs Product Monograph: Key Differences

If you’re bringing a drug or health product to the Canadian market, you’ll need to understand Health Canada’s regulations. Two important terms you’ll come across are Drug Monographs and Product Monographs. While they sound similar, they serve different purposes. Knowing the difference can help you navigate approvals smoothly and avoid delays. In this article, we
... Read moreFDA Fees Summary for 2025

FDA User Fees for Fiscal Year 2025 (FY2025) The FDA’s user fees for FY2025, which runs from October 1, 2024, to September 30, 2025, apply to various industries, including pharmaceuticals, medical devices, biologics, and animal drugs. These fees help support the FDA’s regulatory and review processes, promoting efficient approval and compliance. 1. Prescription Drug User
... Read moreNew Modification to Buffer Zone Affects Food Manufacturers and Suppliers

Simplified Explanation of Changes to Labeling Rules Starting July 2022, food manufacturers and suppliers have been required to display special labeling symbols on certain food products. These include a front-of-package (FOP) nutrition symbol for products high in saturated fat, sugar, or sodium, and a supplemented food caution identifier (SFCI) for supplemented foods with specific health
... Read moreQuality Smart Solutions Launches GRAS Experts to Protect Against Food Safety Risks

GRAS Experts, a new division aimed at tackling ingredient safety concerns and helping businesses stay ahead of regulatory changes and avoiding recalls In light of McDonald’s recent recall of Quarter Pounders due to E. coli contamination, the need for stringent oversight on food ingredients has never been more critical. Quality Smart Solutions has launched GRAS
... Read moreGovernment of Canada Launches Consultation on Plant-Based Egg Alternative Labelling: What Businesses Need to Know

The Canadian Food Inspection Agency (CFIA) has launched a 90-day consultation on proposed guidance for labelling plant-based alternatives to egg products, a critical development for businesses involved in Novel Food Products and ingredients. As the plant-based food market skyrockets, this new consultation presents both an opportunity and a challenge for companies to ensure their products
... Read moreNew Hydration Product Monographs Launched

The NNHPD Opens Consultation on Batch 4 Through new guidelines, stakeholders are invited to shape the future of homeopathic and topical health products. The Natural and Non-Prescription Health Products Directorate (NNHPD) has made a significant announcement, opening a crucial consultation period for Batch 4 of their latest product monographs, including several that will impact the
... Read more7 Must-Know EFSA Novel Food Regulation Changes for 2025: You Need To Be Ready

Are your novel food products ready for the new European Food Safety Authority (EFSA) regulations? If you’re planning to enter the European market in 2025, significant updates to the EFSA novel food regulatory framework are on the horizon, effective from February 1, 2025. These changes will affect everything from risk assessments to labelling, sustainability, and
... Read moreBreaking News: Health Canada’s New GMP Guide for Natural Health Products – Are You Ready?

Health Canada has just released a draft Good Manufacturing Practices (GMP) guide for natural health products (NHPs), marking a monumental shift in how businesses will manufacture, package, label, and store their products. This guide introduces sweeping changes, the first since 2015, to ensure NHPs sold in Canada meet modern safety and quality standards. With updates
... Read moreUnderstanding the Key Changes to Natural Health Product (NHP) Labeling in Canada
Natural health products (NHPs) are widely used in Canada, ranging from vitamins, minerals, and herbal remedies to probiotics and homeopathic medicines. As these products play a vital role in promoting health and wellness, clear and accurate labeling is essential to ensure consumers can make informed decisions about the products they use. To strengthen consumer safety
... Read moreFDA Considerations Selling Foods in USA

Regulatory Considerations when Selling Foods and Beverages in the United States Selling food products in the United States requires compliance with various regulations to ensure consumer safety and public health. The regulatory framework is complex, involving multiple federal agencies, state laws, and industry standards. Here’s an overview of the key regulatory requirements: Federal Agencies:
... Read moreHealth Canada 2024 Changes for Nicotine Replacement Therapies (NRT)

Health Canada 2024 Changes for Nicotine Replacement Therapies (NRT) On August 22, 2024, the Honourable Mark Holland, Minister of Health, announced new measures aimed at preventing youth from being harmed by nicotine replacement therapies (NRTs). The following outlines the recent updates from Health Canada concerning NRTs. There is increasing concern that NRTs, particularly newer formats
... Read moreHow to Create Health Canada Compliant Food Labels

Designing a compliant food label is one of the most important steps when entering the Canadian market. The food labeling requirements in Canada are strict, detailed, and differ significantly from other countries such as the United States. For businesses exporting to Canada, understanding these rules is essential to avoid costly redesigns or product delays. Health
... Read moreNew Update on FDA Sodium Reduction Efforts
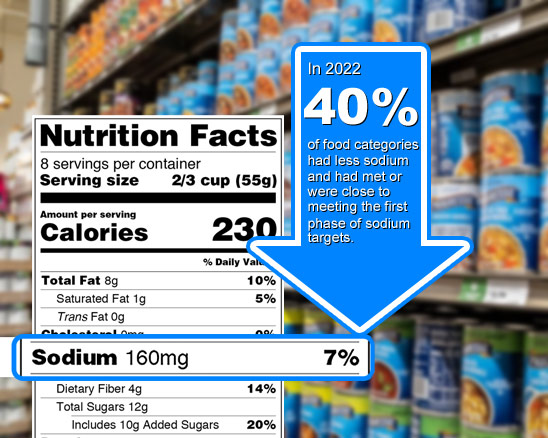
The FDA has taken a significant step forward in its ongoing sodium reduction initiative by issuing new, voluntary sodium reduction targets in a draft guidance, marking the launch of Phase II in their efforts. This follows Phase I, aimed at decreasing diet-related diseases associated with high sodium intake, where final voluntary sodium reduction goals were
... Read moreHealth Canada plans to share document about health products containing CBD this fall

This fall, Health Canada is anticipating sharing a policy consultation document about health products containing (CBD). This proposal has been in the works since 2019. In 2022, Health Canada released a report on health products containing cannabis. Released earlier this year, The Forward Regulatory Plan for 2024 to 2026 details regulatory initiatives that Health Canada
... Read moreFDA MoCRA Compliance Guide for Cosmetic Businesses

Are you struggling to keep up with changing cosmetic regulations? The FDA MoCRA (Modernization of Cosmetics Regulation Act), passed in late 2022, has reshaped how cosmetics are regulated in the U.S., and if you manufacture, distribute, or sell products, these rules now directly impact your business. In fact, MoCRA is the most significant update to
... Read moreHealth Canada’s Regulatory Plan 2024-26: Proposed Amendments to NHP and Non-Prescription Drugs

Health Canada has unveiled its Forward Regulatory Plan for 2024-2026, outlining significant amendments to the Natural Health Products Regulations and the Food and Drug Regulations under the Food and Drugs Act. These amendments are designed to align the regulatory approach for self-care products, ensuring a streamlined and risk-based framework that reflects contemporary standards. To
... Read moreHealth Canada 2024 Drug Establishment Licensing (DEL) Update
Health Canada is introducing a new plan to modernize the Drug Establishment Licensing (DEL) framework over the coming years. This plan, divided into two phases, focuses on strengthening regulatory oversight, aligning with international standards, and easing administrative burdens. Before going into details of the update, let’s throw light on what DEL is and who needs
... Read moreHow to get an NHP Licence for Nicotine Pouches in Canada?

Remember the days of gumming patches, sucking lozenges, and hiding bulky vaporizers? Nicotine pouches, those discreet little packets promising rapid release and sleek satisfaction, are rewriting the playbook. But for NHP manufacturers, the thrill of this pocket-sized revolution comes with a hefty stack of regulatory paperwork. Before you get lost in the regulatory labyrinth, let’s
... Read moreFDA Updates Yogurt Standard: Key Changes for Manufacturers

Attention all yogurt makers, big and small! The U.S. Food and Drug Administration (FDA) has finalized changes to the standard of identity for yogurt, effective January 1, 2024. These updates aim to modernize regulations while maintaining the core characteristics of this beloved food. Here’s the scoop: Yogurt’s getting a modern makeover. The FDA is updating
... Read moreNavigating GRAS Panels: A Guide to Best Practices

When it comes to introducing new substances into the world of food and dietary supplements, ensuring safety is paramount. The GRAS (Generally Recognized as Safe) designation plays a critical role in this process. The U.S. Food and Drug Administration (FDA) provides guidance on how to convene a GRAS panel effectively and responsibly, ensuring that the
... Read moreUnderstanding FDA FURLS, FDA Approved, and FDA Registered Medical Devices
The process of bringing medical devices to market in the United States involves stringent regulations imposed by the Food and Drug Administration (FDA). Understanding the distinction between FDA FURLS (FDA Unified Registration and Listing System), FDA Approved, and FDA Registered is crucial for manufacturers and distributors seeking to comply with these regulations. This article
... Read moreCritical Control Points Explained: The Core of HACCP

https://youtu.be/dVbug89UHBA Introduction Ensuring food safety is crucial for businesses in the food industry. That’s where Hazard Analysis and Critical Control Points (HACCP) comes in, a structured approach that helps prevent contamination and maintain high safety standards. At the center of this system are Critical Control Points (CCPs), key stages in food production where risks must
... Read more