
FDA Updates Yogurt Standard: Key Changes for Manufacturers
Attention all yogurt makers, big and small! The U.S. Food and Drug Administration (FDA) has finalized changes to the standard
... Read more CAN Compliance
CAN Compliance
 USA Compliance
USA Compliance

Attention all yogurt makers, big and small! The U.S. Food and Drug Administration (FDA) has finalized changes to the standard
... Read more
Introduction In the world of medical devices, ensuring patient safety and efficacy is paramount. The 510(k) submission process is a
... Read more
As a business owner operating in dietary supplements and health products, you are undoubtedly aware of the ever-evolving regulatory
... Read more
https://youtu.be/MlufAYFqQ1s In dietary supplements, navigating the intricate regulatory landscape is a challenging yet indispensable aspect of launching your product. Submitting
... Read more
Quality Smart Solutions offers the best FDA Facility Renewal & Registration services in North America for Medical Devices, Food, and
... Read more
Food manufacturers everywhere are willing to jump through hurdles to receive their GRAS Notifications and access to the generally recognized
... Read more
Are you ready to break new ground in healthcare and drug testing? Opening a drug testing facility can be challenging
... Read more
Are you a medical professional or someone involved in the healthcare industry? If so, you’ve likely come across the
... Read more
In today’s global economy, businesses increasingly use international trade to source materials and sell products. However, the complex regulations
... Read more
As a researcher, you know that the success of your study depends on many factors, from the quality of
... Read more
Introduction to Cosmetic Regulations by the FDA: Are you a cosmetics brand looking to navigate the complex world of FDA
... Read more
When thinking about NDI Classification have you ever heard about the New Dietary Ingredient Notification in Ingredient Compliance? In today’s
... Read more
In marketing, the power of persuasive structure-function claims cannot be underestimated. Regarding consumer perception and product sales, structure-function claims have
... Read more
In a world where the pharmaceutical industry is constantly evolving and new drugs are being introduced to the market every
... Read more
Introduction: Welcome to the ultimate guide to conducting a successful clinical trial, where we will delve into the best practices
... Read more
The safety evaluation of direct food additives and color additives used in food will be evaluated in this blog post
... Read more
In today’s competitive marketplace, selling products on Amazon has become a lucrative opportunity for many entrepreneurs and businesses. However, navigating
... Read more
https://youtu.be/iVJlCZmDeWU In today’s highly competitive food industry, ensuring the safety and quality of products is of utmost importance. One key
... Read more
Are you a company in the healthcare or pharmaceutical industry looking to bring a new product to market? If so,
... Read more
Proper documentation is essential in any business operation and is particularly crucial when it comes to PMA submissions. Preparing and
... Read more
The U.S. Food and Drug Administration (FDA) published two final guidelines on March 24, 2023, to help manufacturers of medical devices transition
... Read more
Introduction: When it comes to the pharmaceutical industry, there is no room for error. The production of pharmaceuticals requires strict
... Read more
As more and more people become health-conscious, the demand for supplements has skyrocketed. One of the most popular supplements on
... Read more
Are you running a business that deals with food, drugs, medical devices, cosmetics, or dietary supplements? If so, you must
... Read more
In today’s global food supply chain, ensuring the safety and quality of food products is critical to protecting public health
... Read more
Are you considering getting your food safety certification? In today’s highly competitive food industry, ensuring your food products are safe
... Read more
As our world becomes increasingly globalized, the importation of food has become a common practice. However, with growing concerns
... Read more
https://youtu.be/iVJlCZmDeWU Introduction: In the dynamic realm of food innovation, ushering in new ingredients responsibly and securely is of paramount importance.
... Read more
https://youtu.be/yvnTszySkgg If you’re in the medical device manufacturing business, you’re likely familiar with the FDA’s regulations, including FDA Furls. However,
... Read more
Introduction: On April 13th, the Federal Trade Commission sent letters to warn hundreds of marketers to refrain from misleading customers with product
... Read more
https://youtu.be/NN8ZLynq6kE If you work in the food service industry, you may need to obtain food handlers license to ensure that
... Read more
https://youtu.be/wOKeuDAEfss Have you ever wondered what it means for a food or ingredient to be ‘Generally Regarded as Safe’ (GRAS)?
... Read more
As technology continues to advance, the use of Software as Medical Devices (SaMD) is becoming more prevalent in the
... Read more
BRC certification is a globally recognized standard for food safety and quality management. In Canada, it is becoming increasingly important
... Read more
https://youtu.be/mWaBQzsa1_M Introduction Importing goods can be a complex process, especially when it comes to navigating customs regulations and compliance requirements.
... Read more
Introduction: FDA approvals can be a complex process, but understanding its important steps can give you insight into how drugs
... Read more
As a business owner, keeping up with regulatory requirements is a top priority. One such requirement is the FDA’s renewal
... Read more
Quality Smart Solutions offers the best FDA Facility Renewal & Registration services in North America for Medical Devices, Food, and
... Read more
Introduction to GRAS: Understanding the regulations and safety risks associated with GRAS ingredients can be tricky, but it doesn’t have
... Read more
Introduction: A 510(k) clearance is a process by which medical device manufacturers notify the FDA of their intent to market
... Read more
Introduction: On August 1, 2022, the FDA announced the final guidance on FDA’s policy regarding products labeled as dietary supplements
... Read more
Introduction: The FDA may have turned a blind eye to companies like Bio Steel in the past who’ve been selling
... Read more
Introduction Like most food businesses, you probably spend a lot of time thinking about what’s in your food and how
... Read more
Introduction: The FDA offers an online dietary supplement labeling guide that provides labeling requirements for supplement manufacturers. The guide covers
... Read more
Introduction Prior notice is a requirement for all food manufactured, processed, packed, or held outside the United States that is
... Read more
Introduction Food additives are chemicals added to food products to enhance flavor, texture, or color. Fortunately, there is a list
... Read more
Introduction to GRAS & NDI An ingredient can get into the diet by being a food, food additive, drug, dietary
... Read more
Introduction On November 21, 2022, the Food and Drug Administration (FDA) Center for Food Safety and Applied Nutrition issued warning
... Read more
The Medical Device License is a legal document required to manufacture, sell, and distribute medical devices. The Medical Device
... Read more
Background on FDA NAC Warning Letters: Navigating the FDA’s NAC system can be overwhelming. Understanding FDA warning letters is key
... Read more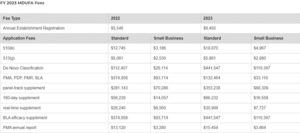
The FDA or U.S. Food and Drug Administration recently announced the Fiscal Year Medical Device User Fee (MDUFA) amendments. The
... Read more
Say you have a new ingredient and are ready to enter the US food market. To ensure this ingredient is
... Read more
Overview Manufacturers of prescription and non-prescription drugs are required to obtain a Drug Identification Number (DIN) before the drug product
... Read more
What is a cosmetic? Under Section 2 of the Food and Drugs Act, a cosmetic is defined as “any substance
... Read more
In response to the COVID-19 public health emergency, FDA issued a declaration regarding the appropriateness of utilizing emergency use authorizations
... Read more
Regulatory agencies across the globe, including the U.S. Food and Drug Administration (FDA), Health Canada, and the United Kingdom’s Medicines
... Read more
The U.S. Food and Drug Administration (FDA) has communicated via Letter of Enforcement Discretion that it will not oppose or
... Read more
Introduction: The Consolidated Appropriations Act, recently passed by the US Congress, incorporates revisions to US cosmetics laws. The Modernization of
... Read more
On November 24, 2021, The Food and Drug Administration (FDA) sent correspondence to industry requesting information on the past use
... Read more
On October 13, 2021, the U.S. Food and Drug Administration (FDA) issued final guidance for the food industry that provides
... Read more
It’s that time of year again! Any domestic and foreign establishments registered with US FDA must renew their registration annually
... Read more
Sesame will become the 9th major food allergen officially recognized by the United States (FDA) on January 1st, 2023. The
... Read more
On Monday September 21st, the U.S. Food and Drug Administration (FDA) announced a proposed rule to establish additional traceability record
... Read moreQuality Smart Solutions is a global consulting firm, engaged in providing strategic support for products including NHPs, pharmaceuticals, and medical devices, in areas such as Regulatory Support, Site Licensing, Clinical Development and more.