The safety evaluation of direct food additives and color additives used in food will be evaluated in this blog post based on recommendations for the minimal toxicity tests to be undertaken.
The details in this FDA guidance document can be used as broad guidelines for figuring out concern levels, the scope, and the types of toxicity testing for added food ingredients and food colorants.
Safety evaluation for a direct food additive background
Using the information on the additive’s predicted toxicological potential from its chemical structure (i.e., low (A), intermediate (B), or high (C)) and an estimation of cumulative human exposure, one can assign the additive to a Concern Level (i.e., low (I), intermediate (II), or high (III). When allocating additives to a Concern Level, exposure information carries more weight than structure alert information. Final safety decisions are made on a case-by-case basis. They may consider additional information when determining the concern level for a food or color additive.
To learn more about the suggested minimum toxicity tests for assessing the safety of indirect food additives, also known as food contact chemicals, now referred to as food contact substances, see Guidance for Industry: Preparation of Food Contact Notifications for Food Contact Substances: Toxicology Recommendations.
Diagram Descriptions
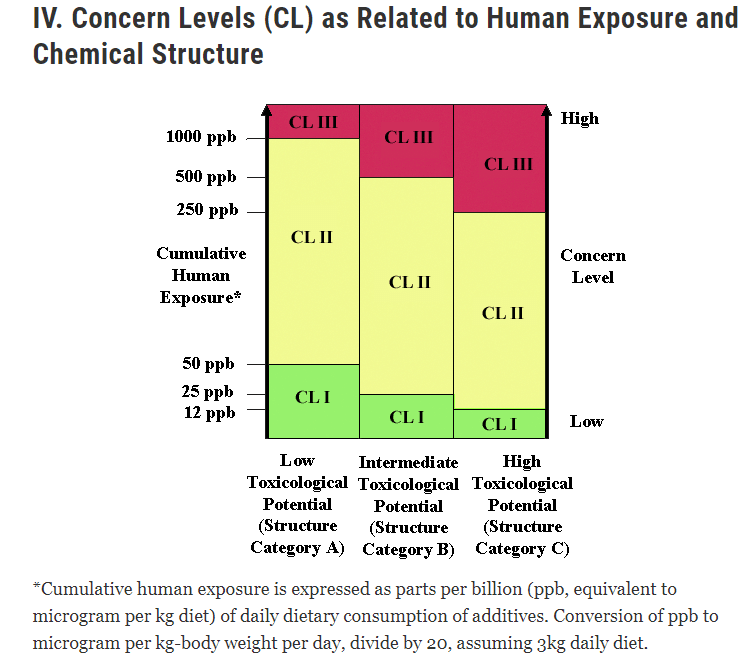
This diagram from the FDA shows the lowest Concern Level that would be given to a direct food additive or food color additive based on the substance’s estimated human exposure from the proposed use and potential toxicity based on structural similarity to known toxicants without toxicological information about an additive. The additive will be classified into one of three broad groups based on the knowledge of its structural characteristics: Category A for minimal toxicological potential, Category B for intermediate potential, and Category C for high toxicological potential. The initial Concern Level to which the additive is given will depend on the expected human exposure within each of the three categories of structures (A, B, and C).
This figure shows recommended breakpoints of exposure that indicate the amount of worry for each structure. The Concern Level equates to the minimum suggested toxicology tests required to assess the toxicological safety of the additive’s new or increased use. I apply concern Level (CL) to Category A structures with cumulative human exposure between 0 and 50 ppb, CL II between 50 ppb and 1000 ppb, and CL III over 1000 ppb. Category B buildings with incremental human exposure levels of 0 to 25 ppb come under CL I, 25 ppb to 500 ppb under CL II, and 500 ppb and higher under CL III. CL I, CL II, and CL III apply to Category C structures with cumulative human exposure between 0 and 12 ppb fall into CL I, CL II from 12 to 250 ppb, and CL III from 250 ppb and above.
The Office of Food Additive Safety, Centre for Food Safety and Applied Nutrition (CFSAN), at the U.S. Food and Drug Administration, has developed this guide with assistance from the Division of Petition Review.
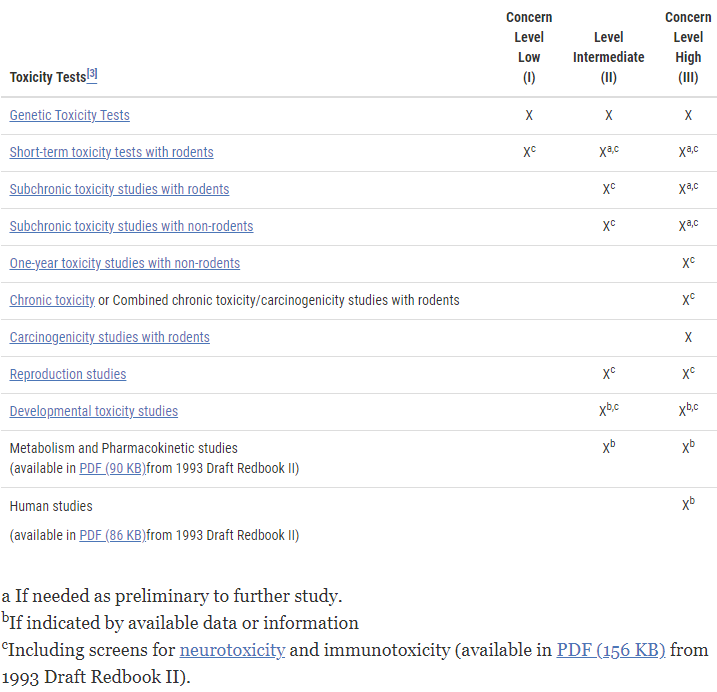
Studies normally indicate that a Concern Level III additive, independent of its chemical structure and exposure, may constitute the minimum toxicological testing to support the safety of a novel additive. To establish the safety of these components, toxicological testing may also be required for metabolites, degradation products, and potential additive contaminants. See below the FDA table on the concern level of various tests that you may need to conduct.