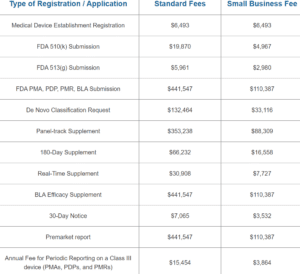
Navigating the Regulatory Landscape: Understanding FDA FURLS, FDA Approved, and FDA Registered Medical Devices
Introduction The process of bringing a medical device to market in the United States involves stringent regulations imposed by the
... Read more