GRAS applications encompass various industries, from food and beverages to pharmaceuticals and cosmetics. By incorporating GRAS ingredients into your products, you can ensure consumer safety and gain a competitive edge in the market. This guide will provide a step-by-step approach to implementing GRAS applications, starting with a detailed overview of what GRAS means and how it is regulated. We’ll then dive into the different industries where GRAS applications are prevalent, exploring the benefits and challenges within each sector.
Understanding the importance of GRAS in various industries
GRAS applications encompass various industries, from food and beverages to pharmaceuticals and cosmetics. By incorporating GRAS ingredients into your products, you can ensure consumer safety and gain a competitive edge in the market. GRAS is crucial as it provides businesses with a framework to determine the ingredients’ safety in various applications. It allows manufacturers to use certain substances without seeking pre-market approval from regulatory bodies, saving time and resources.
In the food and beverage industry, GRAS applications are particularly significant. They ensure that the ingredients used in food products are safe for consumption and do not pose any health risks to consumers. By incorporating GRAS ingredients, food manufacturers can enhance their product offerings and meet the growing demand for safe and healthy food options. Furthermore, GRAS applications in the pharmaceutical, cosmetic, and personal care industries are equally important, as they ensure the safety and efficacy of products used by consumers.
The regulatory landscape for GRAS applications
To fully leverage the potential of GRAS applications, it is crucial to understand the regulatory landscape surrounding them. The United States Food and Drug Administration (FDA) is vital in regulating GRAS applications.
The FDA has established a rigorous process for determining the safety of GRAS ingredients, which involves evaluating scientific evidence and expert opinions. This process ensures that GRAS applications meet the highest security and quality standards. For businesses looking to incorporate GRAS ingredients into their products, navigating the regulatory requirements effectively is essential. Compliance with FDA regulations is crucial to avoid legal issues and maintain consumer trust. Working with regulatory experts and conducting thorough research can help businesses ensure that their GRAS applications are in full compliance with the FDA’s guidelines.
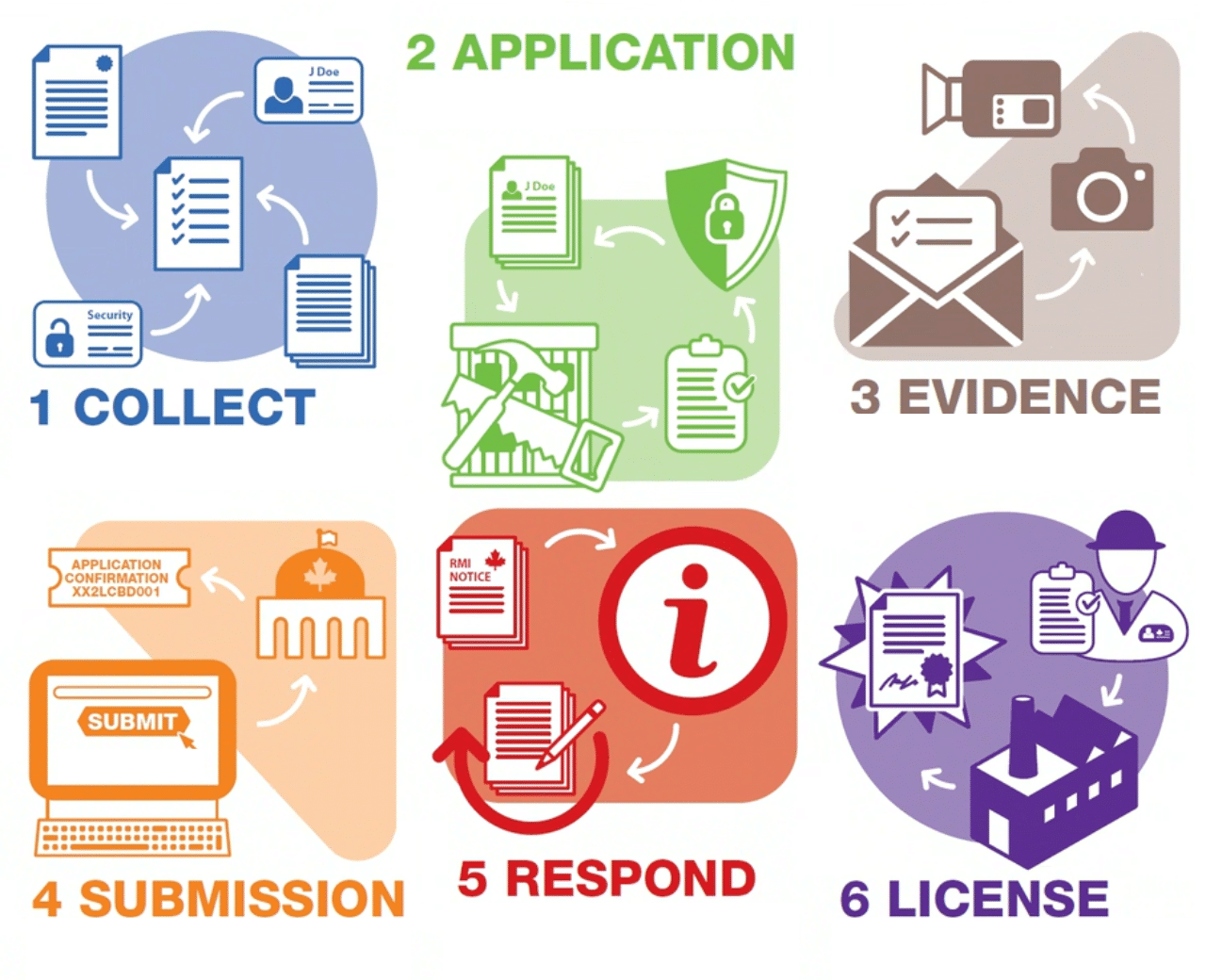
Typical GRAS applications in the food and beverage industry
The food and beverage industry is a prime example of the widespread use of GRAS applications. Many commonly consumed food products contain GRAS ingredients deemed safe for consumption by regulatory authorities. Some examples of GRAS applications in the food industry include natural sweeteners like stevia, preservatives like citric acid, and food colorings like beta-carotene.
GRAS applications in the food and beverage industry offer numerous benefits. Firstly, it allows manufacturers to create products that meet consumer demands for healthier and more natural options. Secondly, GRAS applications provide a level of assurance to consumers, assuring them that the products they consume have undergone rigorous safety evaluations. Finally, incorporating GRAS ingredients can enhance food and beverage products’ flavor, texture, and overall quality.
GRAS applications in the pharmaceutical and healthcare industry
GRAS applications are critical in ensuring the safety and efficacy of medications and healthcare products in the pharmaceutical and healthcare industries. Many active pharmaceutical ingredients (APIs) used in remedies are classified as GRAS, reassuring healthcare professionals and patients. This classification allows pharmaceutical companies to focus on the development and production of medications without the need for extensive safety testing.
The use of GRAS applications in the pharmaceutical industry offers several advantages. Firstly, it streamlines the drug development process, allowing for faster and more cost-effective production of medications. Secondly, it ensures that healthcare products are safe for consumption and do not pose unnecessary patient risks. Finally, GRAS applications in the pharmaceutical industry foster innovation, as researchers can focus on developing new treatments and therapies rather than repeating safety evaluations for existing ingredients.
GRAS applications in the cosmetics and personal care industry
The cosmetics and personal care industry heavily relies on GRAS applications to ensure the safety and effectiveness of their products. Many ingredients used in cosmetics and unique care products, such as moisturizers, shampoos, and makeup, are classified as GRAS. This classification gives manufacturers the confidence to use these ingredients in their formulations without compromising consumer safety.
The use of GRAS applications in the cosmetics and personal care industry has several benefits.
Firstly, it allows manufacturers to create safe skin, hair, and body products. This reassures consumers that their products will not cause harm or adverse reactions. Secondly, GRAS applications in this industry enable the development of innovative and effective products that cater to various consumer needs and preferences. Lastly, incorporating GRAS ingredients can enhance the overall quality and performance of cosmetics and personal care products.
GRAS applications in the agricultural and farming industry
The agricultural and farming industries also benefit from GRAS applications, particularly crop protection and enhancement. Many substances used in agriculture, such as biopesticides and growth enhancers, are classified as GRAS. This classification ensures that these substances do not harm human health or the environment while helping farmers improve crop yields and protect their harvests.
GRAS applications in the agricultural industry offer several advantages. Firstly, they provide farmers with effective and environmentally friendly alternatives to traditional pesticides and fertilizers. This promotes sustainable farming practices and reduces the potential negative impact on ecosystems. Secondly, GRAS applications help farmers meet consumer demands for organic and pesticide-free produce, enhancing market competitiveness. Lastly, they contribute to agricultural products’ safety and quality, ensuring consumers enjoy safe and nutritious food.
Case studies of successful GRAS applications
To further understand the potential of GRAS applications, let’s explore some case studies of successful implementation. One notable example is the use of GRAS ingredients in plant-based meat alternatives. Companies like Beyond Meat and Impossible Foods have successfully developed meat substitutes that taste delicious and provide a safe and sustainable protein source. By using GRAS ingredients, these companies have gained widespread consumer acceptance and disrupted the traditional meat industry.
Another case study is the use of GRAS applications in skincare products. Brands like The Ordinary and Drunk Elephant have created highly effective, popular skincare formulations using GRAS ingredients. These products have garnered a loyal following due to their safety, affordability, and visible results. By leveraging GRAS applications, these brands have revolutionized the skincare industry and set new standards for quality and transparency.
Challenges and considerations in implementing GRAS applications
While the potential of GRAS applications is vast, there are challenges and considerations that businesses must be aware of when implementing them. Firstly, staying up-to-date with regulatory changes and ensuring compliance with evolving guidelines is crucial. The FDA regularly updates its regulations, and businesses must adapt their practices to maintain GRAS status.
Secondly, businesses must invest in research and development to ensure the safety and efficacy of their GRAS applications. Thorough testing and evaluation are necessary to provide scientific evidence supporting the protection of GRAS ingredients. This requires resources and expertise to conduct comprehensive studies and gather relevant data.
Lastly, businesses must effectively communicate the benefits and safety of their GRAS applications to consumers. Transparency and education are vital in building consumer trust and confidence in these products. Clear labeling and marketing efforts that emphasize the safety and quality of GRAS ingredients can help businesses overcome potential skepticism and gain consumer acceptance.
Best practices for maximizing the efficiency and success of GRAS applications
To maximize the efficiency and success of GRAS applications, businesses should follow certain best practices. Firstly, it is crucial to establish strong relationships with regulatory experts who can provide guidance and support in navigating the regulatory landscape. These experts can help businesses understand the requirements and ensure compliance with relevant regulations.
Secondly, investing in research and development is vital to developing innovative GRAS applications. By staying at the forefront of scientific advancements and conducting thorough testing, businesses can create products that meet consumer demands and provide a competitive edge.
Additionally, businesses should prioritize transparency and communication with consumers. Clearly labeling products and providing accurate information about the safety and benefits of GRAS ingredients can build trust and credibility with consumers. Engaging in educational initiatives and sharing scientific research can enhance consumer confidence in GRAS applications.
Resources and tools for navigating GRAS applications
Navigating the world of GRAS applications can be complex, but resources and tools are available to assist businesses. The FDA website provides detailed information on GRAS regulations, guidelines, and the submission process. It is a valuable resource for companies looking to understand the requirements and stay updated on regulatory changes.
Working with regulatory consultants and industry associations can also provide businesses with expert guidance and support. These professionals have in-depth knowledge of GRAS applications and can help enterprises to navigate the regulatory landscape effectively.
Conclusion:
In conclusion, GRAS applications allow businesses to unlock their full potential and achieve peak efficiency and success. By incorporating GRAS ingredients into products, companies can ensure consumer safety, gain a competitive edge, and meet the growing demand for safe and healthy options. Understanding the regulatory landscape, exploring typical applications in various industries, and implementing best practices are crucial for maximizing the benefits of GRAS applications. With the right approach and resources, businesses can harness the power of GRAS and propel themselves forward in their respective industries. Unlock the potential of GRAS applications and unlock a world of possibilities for your business.
We hope this post helped you understand food additives and preservatives a little better. There are many different kinds, and they can have a big impact on your health and the environment. If you want to learn even more about GRAS, reach out to us today about GFSI certification requirements, GRAS Notifications, or GRAS self-affirmation!
Additional Reading about GRAS:
- Gras Certification in the Food Industry
- Navigating the GRAS Process for food manufacturers
- FDA GRAS Status
- GRAS List for Food Additives food safety
- Everything to know about GRAS Ingredients
- Generally Recognized as Safe GRAS Guide
- FDA GRAS Notice & GRAS Database
- NDIN vs GRAS Certification
- GRAS, NDI, ODI Food Additives Unlocked
- Self-Affirmed GRAS Dossier Benefits



