Registering Products with the EPA – What You Need to Know
The US Environmental Protection Agency (EPA) is saddled with the responsibility to register pesticides in the US. This process is a combination of scientific, legal, and administrative procedures through which the EPA examines the ingredients of the pesticide the site or crop where it is to be used the amount, frequency, and timing of its
... Read moreYou submitted your NPN Application what are the next steps?

What to Expect After Submitting an NPN Application In order to sell a Natural Health Product (NHP) in Canada, you require an NPN. NPN stands for Natural Product Number, this is a product license that is issued by the Natural and Non-prescription Health Products Directorate (NNHPD) of Health Canada. The NNHPD oversees all NHP applications
... Read moreHow Pet Food VHP Labels Differ from Human Food Labels in 2022
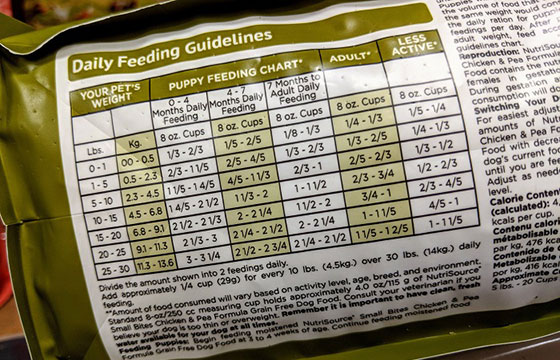
Pet Foods and Human Food Labels are designed to look similar yet distinct. There are some similar label requirements and some that will appear different as they are product specific. Consumer packaging rules in Canada aim to ensure we know what we are buying at time of purchase, so that the consumer can make educated
... Read more
