Pet Foods and Human Food Labels are designed to look similar yet distinct. There are some similar label requirements and some that will appear different as they are product specific. Consumer packaging rules in Canada aim to ensure we know what we are buying at time of purchase, so that the consumer can make educated decisions. Considering common retail set-ups, products will likely be faced the same for both to show main information such as common name and net weight on the front panel and any additional information on the adjacent panels.
Veterinary products are similar to foods by classification as well, in the sense that they are broken into similar-regulated subgroups of foods, drugs and health products.
This blog will highlight several similarities and differences between Pet and Human Food Labels for Canada.
Similarities:
All labels in Canada are required to be Bilingual. French and English are to be equal on all labels as they are both official languages.
The statement or declaration of Net Weight is present on both label types. This allows the consumer to understand how much product is in the container. This can be reviewed against price and serving size at time of purchase.
The Common Name of the product must be listed. This should provide a clear description of the product, be honest and not mislead the consumer with regards to ingredient quantity or effects.
The List of Ingredients must be listed by common name of each component. It also must be listed in descending order by weight. To differentiate for pet foods AAFCO publishes a set of guidelines for the inclusion and list of pet food ingredients.
All Claims and statements are truthful and not misleading. All call outs and claims must be accurate and honest and supported by verifiable evidence. Deceptive awards and endorsements must not be used. Comparison to other products and companies is not suggested.
All labels should clearly indicate the Company Name and Address. This allows the consumer to contact the product owner to report any side effects, leave a review or ask additional questions.
Differences:
Rather than a nutrition facts table, pet foods employ and are required to list a table of Guaranteed Analysis. This explains the total crude protein, crude fat, crude fibre, and moisture within the product. Like NFTs on a human food, this can be compared to other pet foods when trying to decide between two or more products.
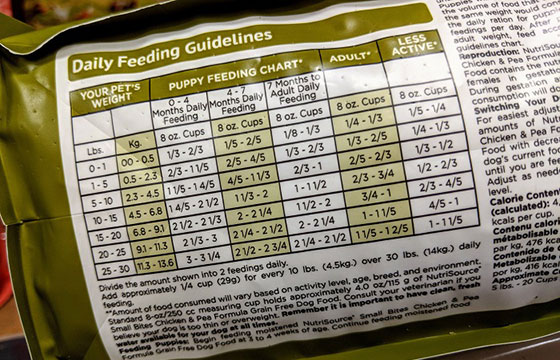
Pet foods must list feeding instructions. This piece can also refer to the product being able to be used as a feed or a supplemental feed. This directional item must be called out on the label. If the product is intended for specific dietary use, the direction to use as recommended by a veterinarian may be required. Human foods often list preparation instructions but not directions as they are often thought of for ad-libitum consumption.
Pet Foods must list the nutritional adequacy or intended life stage for the product. If the product has passed a feeding test or has specific nutrient values for a specific life stage. Then this can be explained on the label. Human foods are not usually labelled for a specific demographic, this may be part of the marketing campaign, but not overly apparent on the label.
The Product Identity will need to be made clear. Whether the product is intended for Dogs or Cats. This should be depicted in writing. Often pet foods have graphics/images that show the intended animal.
Summary:
In conclusion there are many differences and similarities between Pet and Human Food labels, most of which are apparent if you were to hold the two side by side.
Please reach out to our team of specialists to further discuss your human or pet food products. We are happy to help discuss classification, labelling and registration for your products!
References
https://www.competitionbureau.gc.ca/eic/site/cb-bc.nsf/eng/01229.html



