
How Audits Help You Stay Compliant with Health Canada
When it comes to audits related to Health Canada requirements, staying compliant is about more than checking off a list.
... Read more
When it comes to audits related to Health Canada requirements, staying compliant is about more than checking off a list.
... Read more
Starting June 9, 2025, Class II NHP submissions will no longer be reviewed just based on the date you filed them. Health Canada is updating how it handles licensing applications by applying a new workload management approach, prioritizing submissions based on intent to sell or manufacture in Canada.
... Read more
Understand how to stay compliant with FDA and Health Canada rules for eczema products. Learn how to properly classify and register your product.
... Read more
Health Canada is conducting a public consultation on CBD regulations for NHPs, exploring a pathway to allow non-prescription CBD products under the NHPR. This consultation opened on March 7, 2025, and will close on June 5, 2025.
... Read more
Health Canada is prioritizing Class III NHPs made or sold in Canada. Learn what this means for your application and how to stay compliant.
... Read more
Are you looking to bring your natural health product to market? Navigating the process of obtaining a license can be
... Read more
Are you a business owner in the health and wellness industry? If so, you’re likely familiar with Health Canada’s Natural
... Read more
https://youtu.be/mWaBQzsa1_M If you’re a business owner looking to expand your reach into the Canadian market, importing products may be a
... Read more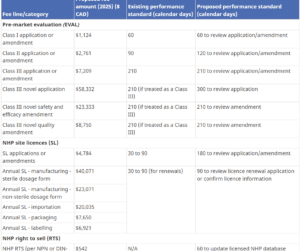
Health Canada has the authority to set and charge health product fees under the Food and Drugs Act (FDA). On
... Read more
The Licensing and Non-paired Health Products Directorate (LNHPD) is a federal government organization that oversees the regulation of natural health
... Read more
The Canadian government has a set of regulations in place for natural health products (NHPs). These regulations help to
... Read more
https://youtu.be/O7GbA-Awo0g 1. What is the NHP Licensing Process/what is needed for it? This process involves the submission of a product
... Read more
https://youtu.be/veCBMkYUWEU Market Analysis and Overview of CPG Market: Will CBD Products be considered an NHP? The consumer packaged goods (CPG)
... Read more
Who needs a Foreign Site Reference Number (FSRN)? For Natural Health Products (NHPs) manufactured, packaged, and/or labeled by sites located
... Read more
Learn how to classify your product as a drug, NHP, or cosmetic in Canada. Understand key decision-making criteria, borderline examples, and what regulatory path you need to follow to stay compliant with Health Canada.
... Read more
Top 5 Tips to Successfully Secure an NPN Number In order to sell a Natural Health Product (NHP) in Canada
... Read more
Natural Health Products have different characterization, identification, quantification, and purity standards that you should know about to ensure the quality
... Read more
All Natural Health Products (NHPs) sold in Canada are subject to the Natural Health Products Regulations (NHPR). Natural Health Products
... Read more
The classification of Foods and Natural Health Products (NHPs) can be considered difficult, grey, and convoluted. Sometimes even comes down
... Read more
What to Expect After Submitting an NPN Application In order to sell a Natural Health Product (NHP) in Canada, you
... Read more
In March 2020, Health Canada introduced interim measures to expedite the issuance of site licences for alcohol-based hand sanitizers, to
... Read more
How to Sell Dietary Supplements or NHPs on Amazon or Other Online Platforms in Canada? What is an NHP? In
... Read more
Natural Health Products (NHPs) are regulated by Health Canada under the authority of the Food and Drugs Act and the Natural
... Read more
The coronavirus-19 (COVID-19) pandemic has had a long-lasting effect on many of the processes done in business and everyday life.
... Read more
Introduction: Identifying the correct application type has become increasingly crucial for Natural Health Product (NHP) applications. Incorrect classification can result
... Read more
Natural Health Product Brand Name Guidance Brand names are an important part of your marketing strategy, but as many of
... Read more
In September Amazon.ca notified every Natural Health Product vendor (whether they had an NPN or not) that, effective in October
... Read more
Supplemented foods and natural health products (NHPs) can sometimes pose challenges when trying to determine the correct regulatory pathway to
... Read more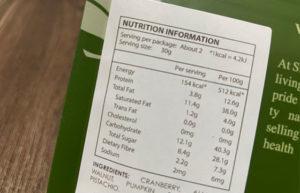
Health Canada implemented the Plain Language Labelling (PLL) initiative to help make the labels for non-prescription health products easier to
... Read moreQuality Smart Solutions is a global consulting firm, engaged in providing strategic support for products including NHPs, pharmaceuticals, and medical devices, in areas such as Regulatory Support, Site Licensing, Clinical Development and more.
|
|
Thank you for Signing Up |
