Navigating the complex medical device regulatory process can often feel like decoding a cryptic language. Manufacturers grapple with an intricate web of requirements, standards, and timelines while striving to bring life-saving innovations to market. But fear not! This comprehensive guide aims to demystify the medical device regulatory process and empower manufacturers with the knowledge and insights to navigate this challenging landscape successfully.
From understanding the different regulatory bodies and their roles to deciphering the essential documentation and testing requirements, this guide is your go-to resource for unraveling the intricacies of medical device regulations. We’ll also highlight crucial milestones and best practices to ensure a smooth and efficient regulatory pathway for your product. So, whether you’re a seasoned manufacturer looking to refresh your knowledge or a newcomer, join us as we unravel the mysteries and unlock the secrets of the medical device regulatory process.
Importance of regulatory compliance for Medical Device Manufacturers
Ensuring regulatory compliance is of utmost importance for medical device manufacturers. Compliance with regulations guarantees the safety and efficacy of the devices and instills confidence in both healthcare professionals and patients. Non-compliance can lead to severe consequences, including product recalls, fines, legal liabilities, and brand reputation damage. Regulatory compliance is a legal requirement and a critical aspect of responsible business practices in the healthcare industry.
Manufacturers must understand and comply with the regulations set forth by various regulatory bodies and agencies to gain market access to their medical devices. These regulations protect patient safety, ensure device effectiveness, and promote public health. By adhering to these regulations, manufacturers can demonstrate their products’ quality, safety, and performance, ultimately leading to successful market entry.
Regulatory compliance ensures manufacturers keep up with the latest industry standards and advancements. By staying updated with evolving regulations, manufacturers can adapt their processes, materials, and technologies to meet the changing requirements. This helps maintain compliance and fosters continuous improvement and innovation in the medical device industry.
Regulatory bodies and agencies involved in the process
The medical device regulatory landscape involves multiple regulatory bodies and agencies, each with responsibilities and jurisdictions. Understanding the roles and functions of these entities is crucial for manufacturers seeking regulatory approval for their devices.
1. Food and Drug Administration (FDA): In the United States, the FDA is the primary regulatory body responsible for ensuring the safety and effectiveness of medical devices. The FDA evaluates and approves devices before they can be legally marketed in the country. They review pre-market submissions, conduct inspections, and monitor post-market surveillance to ensure regulation compliance.
2. European Medicines Agency (EMA): The EMA plays a vital role in regulating medical devices in the European Union. They assess devices’ safety, quality, and performance through rigorous evaluation. The EMA also collaborates with national competent authorities to ensure consistent implementation of regulations across member states.
3. International Medical Device Regulators Forum (IMDRF): The IMDRF is a global forum with regulatory authorities from different countries. It aims to harmonize medical device regulations and promote international cooperation. The IMDRF develops guidelines and standards to facilitate regulatory convergence and reduce barriers to market access.
4. Other Regulatory Bodies: Apart from the FDA and EMA, many countries have regulatory bodies responsible for oversight of medical devices. For example, Health Canada in Canada, the Therapeutic Goods Administration (TGA) in Australia, and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan. Manufacturers must familiarize themselves with the specific requirements and processes of the regulatory bodies relevant to their target markets.
Understanding the roles and expectations of these regulatory bodies is essential to navigate the regulatory process for medical devices successfully. Manufacturers should establish clear lines of communication with the relevant authorities and seek their guidance throughout the regulatory journey.
Classification of medical devices and its impact on the regulatory process
Medical devices are classified into different categories based on their risk level. The classification of a device determines the level of regulatory scrutiny it undergoes and the requirements for approval. The classification criteria may vary between regulatory bodies but typically include factors such as intended use, potential risks, and duration of device contact with the body.
Commonly, medical devices are classified into three main categories: Class I, Class II, and Class III. Class I devices are considered low-risk and are subject to fewer regulatory controls. Class II devices pose a moderate risk and require more stringent regulatory oversight. Class III devices, on the other hand, are high-risk and undergo the most rigorous regulatory scrutiny.
The classification of a device has a significant impact on the regulatory process. Class I devices usually do not require pre-market approval. They can be marketed after fulfilling general controls and registration requirements. Class II devices often require pre-market notification or 510(k) clearance, which involves demonstrating substantial equivalence to a predicate device. Class III devices typically require pre-market approval, which involves comprehensive clinical data and rigorous testing to demonstrate safety and efficacy.
Manufacturers need to classify their devices to determine the appropriate regulatory pathway accurately. A thorough understanding of the classification criteria and associated requirements is crucial for successful market entry.
Pre-market requirements for Medical Device Manufacturers
Manufacturers must fulfill pre-market requirements before a medical device can be legally marketed. These requirements are designed to ensure that devices meet the necessary standards for safety, effectiveness, and performance.
1. Quality Management System (QMS): Manufacturers must implement a robust QMS to ensure their devices are consistently manufactured under regulatory requirements. A QMS encompasses processes, procedures, and documentation that govern medical device design, development, manufacturing, and distribution.
2. Design and Development: Manufacturers must establish a systematic design and development approach, including risk management, design controls, and verification/validation activities. Design inputs, outputs, and documentation should be well-documented and traceable.
3. Clinical Evaluation: Clinical data is essential for higher-risk devices to demonstrate safety and efficacy. Manufacturers must conduct clinical evaluations to assess the device’s performance and collect relevant data. Clinical investigations may be required to generate additional evidence, especially for novel devices or those with higher risk profiles.
4. Labeling and Instructions for Use: Proper labeling is crucial for communicating important information to users and ensuring the safe and effective use of the device. Manufacturers must provide clear and accurate labeling, including indications for use, contraindications, warnings, and precautions.
5. Pre-market Submissions: Depending on the device classification, manufacturers may need to submit pre-market notifications, 510(k) applications, or pre-market approval applications. These submissions should include comprehensive documentation, such as device descriptions, performance data, labeling, and clinical evidence.
By fulfilling these pre-market requirements, manufacturers can demonstrate their devices’ safety, effectiveness, and quality, paving the way for regulatory approval and market entry.
Clinical Trials and data requirements for Medical Device Approval
Clinical data is crucial for regulatory approval for medical devices with higher risks or that lack substantial equivalence to predicate devices. Clinical trials are vital for generating evidence to support device safety and efficacy claims. The level of clinical evidence required depends on the device’s classification and the potential risks associated with its use.
1. Clinical Investigation Planning: Manufacturers planning to conduct clinical investigations must develop a comprehensive plan to meet the study objectives. The project includes study design, patient selection criteria, endpoints, statistical analysis, and ethical considerations.
2. Ethical Considerations: Manufacturers must obtain ethical approvals from appropriate review boards or ethics committees before initiating clinical investigations. Ethical considerations ensure the protection of patient rights, minimize risks, and uphold the principles of medical research.
3. Patient Recruitment and Informed Consent: Manufacturers must recruit suitable patients who meet the clinical investigation’s defined inclusion and exclusion criteria. Informed consent, a crucial aspect of ethical research, ensures that patients are fully informed about the study, its risks and benefits, and their rights before participating.
4. Data Collection and Analysis: Manufacturers collect data on device performance, patient outcomes, and adverse events during the clinical investigation. The data should be ordered according to predefined protocols and analyzed using appropriate statistical methods to draw valid conclusions.
5. Clinical Evaluation Report (CER): A CER summarizes the clinical data collected during investigations and assesses the device’s safety and performance. The CER is essential to the regulatory submission, providing evidence of clinical benefits and supporting the device’s claims.
Manufacturers can gather the necessary data to support their device’s safety and effectiveness claims by conducting well-designed and executed clinical trials. This data is crucial for regulatory approval and market access. Manufacturers should work closely with healthcare professionals, clinical research organizations, and regulatory bodies to ensure compliance with clinical data requirements.
Post-market Surveillance and Reporting Obligations
Post-market surveillance is a critical aspect of the medical device regulatory process. Once a device is on the market, manufacturers must monitor its performance, detect potential issues, and take appropriate actions to ensure patient safety.
1. Adverse Event Reporting: Manufacturers must establish systems to monitor and report adverse events related to their devices. Adverse events include any unexpected or unwanted effects caused by the device, including malfunctions, injuries, or deaths. Timely reporting of adverse events is crucial for identifying potential safety concerns and initiating corrective actions.
2. Field Safety Corrective Actions (FSCA): When a device poses a risk to patient safety, manufacturers must implement FSCA to address the issue. FSCA may include device recalls, repairs, modifications, or other actions to mitigate the risk. Manufacturers must promptly communicate with regulatory authorities, healthcare professionals, and users to ensure the necessary measures are taken.
3. Post-market Clinical Follow-up (PMCF): Manufacturers may be required to conduct post-market clinical follow-up studies to gather additional data on device performance and safety. PMCF studies help identify long-term effects, rare adverse events, and device performance in real-world settings. The findings from PMCF studies contribute to ongoing device surveillance and may lead to further improvements.
4. Trend Analysis and Surveillance: Manufacturers should establish systems to analyze and monitor trends related to device performance, complaints, and adverse events. Trend analysis helps identify patterns, potential risks, and areas for improvement. By proactively tracking trends, manufacturers can take early action to address any emerging issues.
Manufacturers can ensure their devices’ continued safety and effectiveness by monitoring and reporting post-market data. Collaboration with regulatory authorities, healthcare professionals, and users is essential to maintaining a robust post-market surveillance system.
Common challenges faced by manufacturers during the regulatory process
The medical device regulatory process poses several challenges for manufacturers. Understanding and proactively addressing these challenges can help manufacturers navigate the process more effectively.
1. Navigating Complex Regulations: The sheer number of regulations, guidelines, and standards can overwhelm manufacturers. Staying current with the latest requirements and ensuring compliance across multiple markets can take time and effort. Manufacturers must invest time and resources in understanding the specific regulations applicable to their devices and seek expert guidance when needed.
2. Resource Constraints: The regulatory process requires significant resources, including time, personnel, and financial investment. Manufacturers may need help allocating these resources, especially for small or new companies. Adequate planning, resource allocation, and collaboration with external experts can help overcome resource constraints.
3. Clinical Data Requirements: Generating clinical data can be time-consuming and costly. Collecting sufficient data to meet regulatory requirements, especially for high-risk devices, can pose challenges. Manufacturers should carefully plan and execute clinical trials, collaborate with research organizations, and explore innovative approaches to streamline data collection.
4. Harmonization and Convergence: The need for harmonization and convergence among regulatory bodies can create challenges for manufacturers seeking global market access. Different requirements, timelines, and processes across regions can lead to delays and increased costs. Manufacturers should proactively engage with regulatory bodies, participate in harmonization initiatives, and leverage regulatory expertise to navigate these challenges.
5. Changing Regulatory Landscape: Regulatory requirements and expectations constantly evolve. Keeping up with these changes and adapting processes can be challenging for manufacturers. Staying informed, engaging with regulatory bodies, and maintaining a culture of continuous improvement can help manufacturers address these challenges effectively.
By acknowledging and addressing these challenges, manufacturers can navigate the regulatory process more efficiently and minimize potential roadblocks to market entry. Collaboration with regulatory experts, industry associations, and other stakeholders can provide valuable insights and support throughout the process.
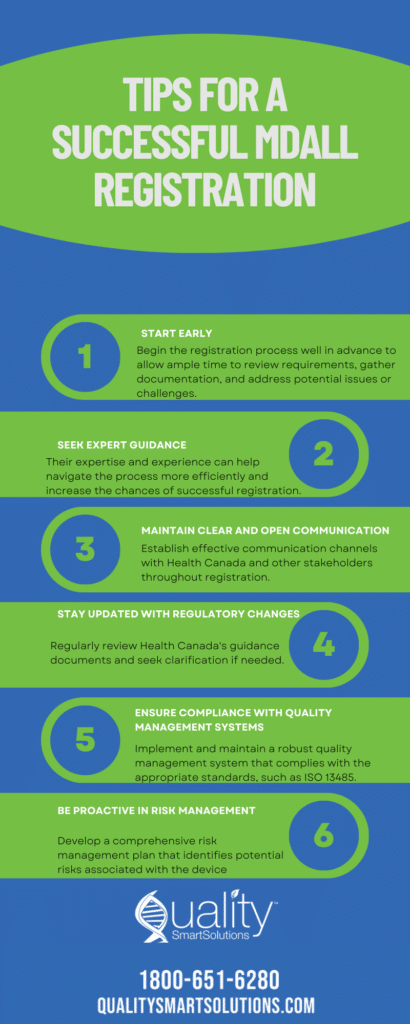
Steps to streamline the regulatory process for Medical Devices
While the medical device regulatory process can be complex, manufacturers can take specific steps to streamline the process and ensure a smooth pathway to market entry.
1. Early Regulatory Strategy: Develop a comprehensive regulatory strategy early in the device development process. This strategy should consider the target markets, device classification, regulatory requirements, and timelines. Early engagement with regulatory bodies and seeking guidance can help align the plan with regulatory expectations.
2. Thorough Documentation: Maintain accurate and comprehensive documentation throughout the device development and regulatory process. Well-documented design history files, clinical data, testing reports, and labeling information are crucial for regulatory submissions and audits. Clear and organized documentation streamlines the review process and reduces potential delays.
3. Collaboration with Regulatory Experts: Engage with regulatory experts, consultants, and legal advisors specializing in medical device regulations. Their expertise can help navigate the complexities, interpret rules, and provide guidance throughout the regulatory journey. Collaborating with external experts can save time and resources and ensure compliance with the latest regulatory requirements.
4. Effective Risk Management: Implement a robust risk management process encompassing risk assessment, mitigation, and monitoring throughout the device lifecycle. Identifying and addressing potential risks early in the development process minimizes the chances of regulatory hurdles and enhances patient safety.
5. Pre-submission Meetings: Take advantage of pre-submission meetings with regulatory bodies to discuss the device, its intended use, and the regulatory requirements. These meetings provide an opportunity to seek clarification, address concerns, and ensure alignment between the manufacturer and the regulatory authority.
6. Continuous Monitoring and Improvement: Establish a post-market surveillance system to monitor device performance, complaints, and adverse events. Regularly review and analyze the data to identify trends, potential risks, and opportunities for improvement. Proactive monitoring allows manufacturers to take prompt action when necessary and maintain compliance with post-market obligations.
By following these steps, manufacturers can streamline the regulatory process, reduce time to market, and ensure
Our experts at Quality Smart Solutions are here to help and offer medical device-related regulatory advice and support on successfully securing your medical device license. We can help you by responding to potential information requests, keeping your license updated, and reviewing your device labels (510k Medical Device Registration, Facility Registration & FURLS, IVD Device Registration, and SaMD Classification.




 Introduction
Introduction
 Introduction: Navigating international trade regulations and the intricacies of Importer of Record (IOR) compliance and taxes can be complex. This comprehensive guide breaks down IOR compliance and its connection to tax obligations in the USA, offering a clear understanding for businesses engaged in global trade.
Introduction: Navigating international trade regulations and the intricacies of Importer of Record (IOR) compliance and taxes can be complex. This comprehensive guide breaks down IOR compliance and its connection to tax obligations in the USA, offering a clear understanding for businesses engaged in global trade.
 Food labels are more than just ingredient stickers on packaging; they are a key tool that helps consumers make informed choices about what they eat. This guide simplifies the complex world of food labeling regulations, focusing on the rules set by Health Canada, the U.S. Food and Drug Administration (FDA), and the European Union (EU). From required elements to health claims and country-of-origin labeling, this article breaks down the essential information you need to know about food labels and their purpose.
Food labels are more than just ingredient stickers on packaging; they are a key tool that helps consumers make informed choices about what they eat. This guide simplifies the complex world of food labeling regulations, focusing on the rules set by Health Canada, the U.S. Food and Drug Administration (FDA), and the European Union (EU). From required elements to health claims and country-of-origin labeling, this article breaks down the essential information you need to know about food labels and their purpose.
 Del Health Canada is a crucial regulatory body responsible for ensuring the safety and effectiveness of drugs and medical devices in Canada. This organization plays a vital role in protecting the health and well-being of Canadians by setting standards, conducting research, and enforcing regulations. In this guide, we’ll explore the responsibilities and functions of Del Health Canada in more detail.
Del Health Canada is a crucial regulatory body responsible for ensuring the safety and effectiveness of drugs and medical devices in Canada. This organization plays a vital role in protecting the health and well-being of Canadians by setting standards, conducting research, and enforcing regulations. In this guide, we’ll explore the responsibilities and functions of Del Health Canada in more detail.







 Are you ready to take your business to new heights? Look no further. In this comprehensive guide, we will unlock the potential of GRAS applications to help you achieve peak efficiency and success. Whether you’re a small startup or an established enterprise, understanding the power of GRAS (Generally Recognized as Safe) applications is essential for reaching your business goals.
Are you ready to take your business to new heights? Look no further. In this comprehensive guide, we will unlock the potential of GRAS applications to help you achieve peak efficiency and success. Whether you’re a small startup or an established enterprise, understanding the power of GRAS (Generally Recognized as Safe) applications is essential for reaching your business goals.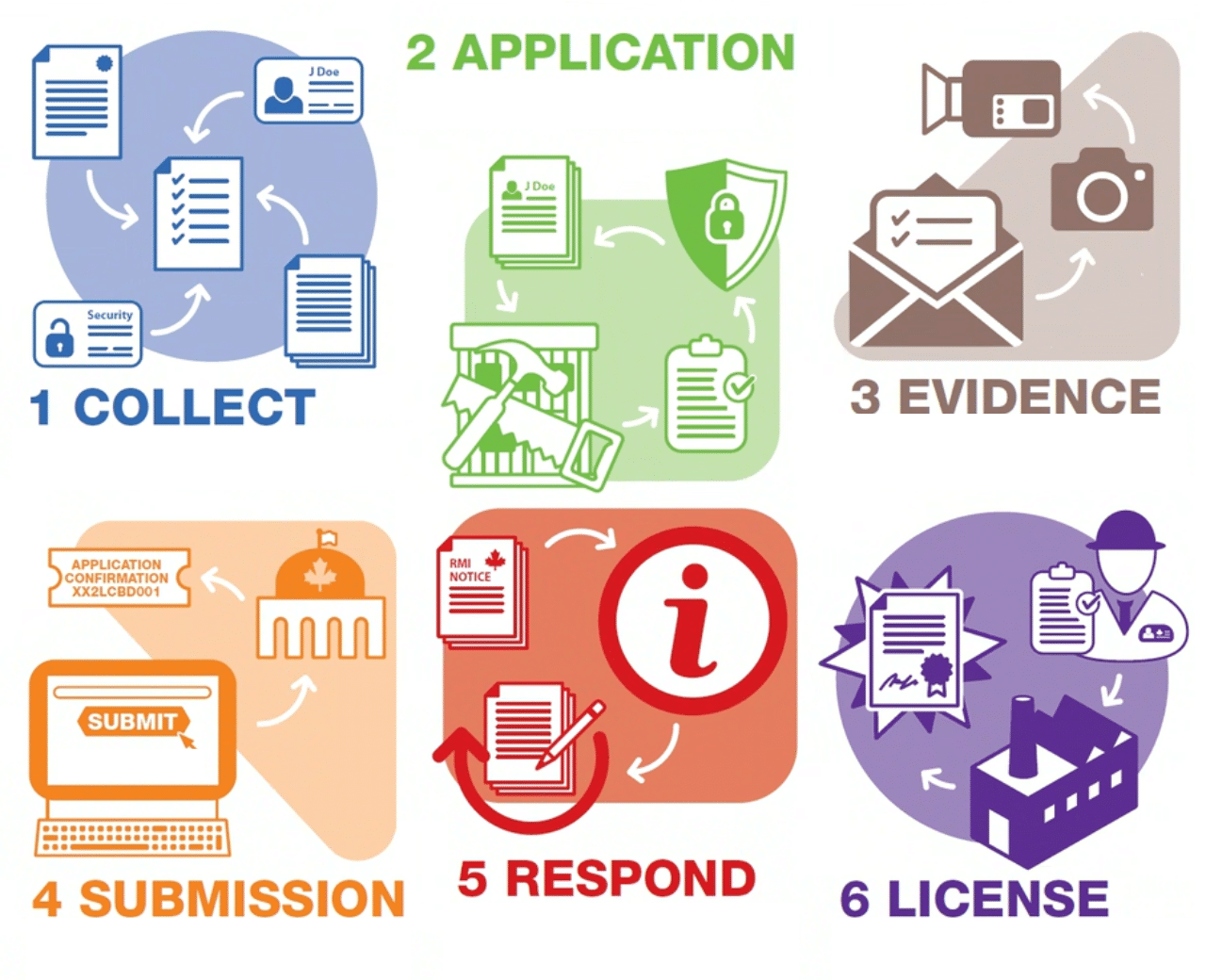





 Introduction:
Introduction:


 Food manufacturers everywhere are willing to jump through hurdles to receive their
Food manufacturers everywhere are willing to jump through hurdles to receive their 






 In today’s rapidly evolving regulatory landscape, businesses face many challenges regarding ensuring compliance and product safety. One crucial aspect often overlooked is the importance of a
In today’s rapidly evolving regulatory landscape, businesses face many challenges regarding ensuring compliance and product safety. One crucial aspect often overlooked is the importance of a