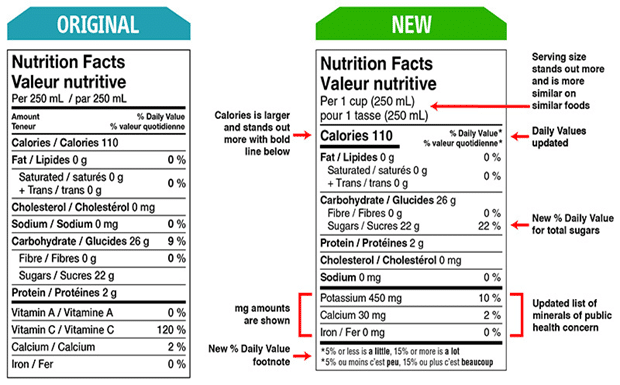
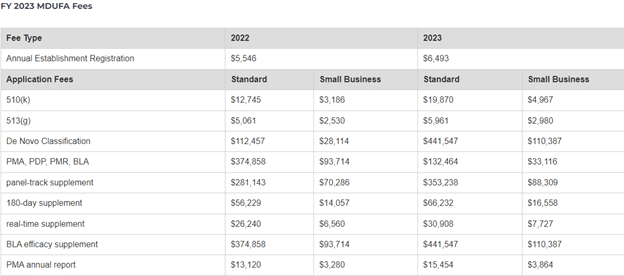
HACCP, which stands for Hazard Analysis and Critical Control Points, is the cornerstone of food safety plans in the food industry. It is a concept designed to identify and prevent or eliminate any potential hazards that may put consumers at risk of food-borne illnesses. This guide will take an in-depth look into what HACCP is and how it can be used to ensure food safety and quality.
When it comes to food safety, compliance is a top priority for any business. And that means consulting with the right experts to ensure your operations are up to code. The company has years of experience helping businesses navigate the complex world of food safety regulations.

This blog post will look at 7 HACCP principles every food safety consultant should know. These principles will help you understand your clients and work more effectively with them to ensure compliance.
How to set up a HACCP Plan?
Setting up a HACCP plan is an important first step when becoming certified or licensing your business. This plan will help identify potential hazards and create a process to reduce the risk of foodborne illness. Many software applications can help you create your HACCP plan but choosing one that is comprehensive and easy to use is important.
The Food Safety Modernization Act (FSMA) requires all food businesses with 20 or more employees to have a written HACCP plan by January 1, 2017. If you are not already licensed or certified, getting started on your HACCP plan as soon as possible is important. There are a number of resources available online and through your state Extension office that can help guide you through the process.
Once you have created your HACCP plan, you must implement it correctly. A good way is to conduct regular “sanitation audits” of your operations.
What is HACCP?
HACCP is a systematic approach to identifying, assessing, and controlling hazards in food operations. It is a globally accepted method for preventing safety risks associated with the production of foods. HACCP works by requiring food producers to identify potential risks from a holistic point-of-view and then develop a plan to address those risks. The goal is to make sure that all control points in the food production process are identified and monitored so that there is minimal risk from hazards such as contamination or spoilage.
Regulatory Consulting HACCP 7 Principles Unlocked
1. Identify and analyze hazards (Principle # 1)
Hazard identification and analysis are essential to developing a safe food safety plan. The first step in hazard identification is to identify potential food safety hazards. To do this, you need to understand the basic principles of hazard analysis.
The first principle of hazard analysis is that all hazards are related. It means that if you identify one risk factor, you’re likely to also identify other associated risks. For example, if you’re looking at the potential for bacteria contamination, you’ll need to consider heat exposure, cross-contamination, and moisture levels.
The second principle of hazard analysis is that all hazards can cause food safety problems. It means that no matter how small the risk may seem, it can still lead to an unsafe product if it’s not properly addressed. For example, even a very small number of Listeria monocytogenes cells can cause serious illness in people who eat contaminated food.
The final principle of hazard analysis is that food safety risks can vary depending on the situation. It means that different risks might be more or less likely to occur in different scenarios, such as during production (when there’s a high level of contamination) or during distribution (when products reach consumers).
2. Determine the Critical Control Points (CCPs) (Principle # 2)
When determining the CCPs for a food production facility, there are many things to consider. The key is to identify points where contamination could easily occur and take action to prevent it.
The following tips will help you determine the CCPs:
- Look at the layout of the facility and make a list of potential contamination points. These include where raw materials or finished products are handled, workers enter and exit the facility, or equipment is maintained.
- Evaluate how easily contaminants could enter or leave the facility. For example, if there are many entrances and exits, contaminants may be less likely to stay inside the facility. If there are few points of entry, then contaminants may be more likely to spread throughout the facility.
- Consider how cleanliness affects CCPs. For example, if bacteria can live on surfaces for a short time, then they may be able to contaminate food products during manufacturing or storage. Similarly, dirty equipment can create conditions that allow spoilage and infection.
3. Establish Critical Limits for each CCP (Principle # 3)
The third CCP principle is establishing Critical Limits. Critical limits are the maximum amount of contaminants or pollutants that CCPs can release into the environment. By reaching and keeping critical limits, the CCPs work as they should and do not pose an unacceptable risk to human health or the environment.
A company must first identify the contaminants or pollutants that comprise the CCPs’ emission profile to establish critical limits. Once the profile is known, a company can use mathematical models to determine the allowable levels of each contaminant in air, water, or soil. The allowable levels should be set at a level that protects human health and the environment while allowing for normal operations of the CCPs.
Setting critical limits is a complex process that requires careful consideration of all factors involved in emissions from a CCP. It is important to remember that critical limits may change over time as new information becomes available about how contaminants interact with each other and with humans and the environment. Therefore, it is important for companies to regularly review their critical limit settings and make any necessary adjustments.
4. Establish a Monitoring Procedure (Principle # 4)
There are a few things that you should consider when establishing your monitoring procedure in order to ensure compliance with HACCP principles.
You first need to decide what type of monitoring you will do. You can choose to monitor either the process or the product. The choice depends on which area of concern most concerns you.
If you are concerned about the quality of the product, then you should focus on monitoring the process. It means focusing on things like cleanliness, sanitation, and hygiene. You should also track how often products are produced and tracked throughout their life cycle.
Need FSVP Assistance or help Creating a HACCP Plan?
5. Establish Corrective Actions (Principle # 5)
Develop corrective actions for identified deficiencies in safety performance.
A regulatory consulting firm must be able to identify and correct deficiencies in safety performance, regardless of the cause. Identifying and correcting deficiencies takes a comprehensive approach that includes inspection, review of records, interviews, and data analyses. Once the deficiencies are identified, consultants should develop corrective actions to ensure that operations continue to meet applicable safety standards.
6. Verify the HACCP Plan (Principle # 6)
HACCP verification is important in ensuring that your food safety plan is effective. The verification process helps to ensure that all key elements of your HACCP plan are in place and operational. It also allows you to identify any plan deviations and determine if corrective action is necessary.
One of the most important aspects of verification is verifying the holding temperature of food. This parameter should be held at a temperature that will prevent spoilage and should not exceed 41 degrees Celsius (105 degrees Fahrenheit). If the food is not held at this temperature, it may be subject to microbial growth, resulting in foodborne illness.
7. Keep Records (Principle # 7)
Keeping records is the key to compliance and ensuring regulatory compliance. By keeping records, you can ensure that all relevant information is captured and preserved to allow easy retrieval during an audit or inquiry.
There are a few different ways to keep your records: written, electronic, or both. The most important factor is having a system that allows you to easily capture and track information. You should also make sure that all records are accurate and up to date.
Record keeping is an important aspect of the HACCP plan due to the following:
- Offers traceability and transparency
- Ensures due diligence
- Provides a record of complaints with critical limits set
- Identifies potential problems
You should also maintain detailed logs of all interactions with regulators, including phone calls, e-mails, meeting minutes, and correspondence. This information will help you reconstruct what occurred during any interaction and remember any pertinent details.
How to Implement the HACCP System in Your Food Facility:
Once you have all the basics in place, there are certain steps to take when beginning to establish and implement your HACCP system. First, you must create a HACCP team, which should include people knowledgeable about the process and familiar with the equipment and production environment. After that, your team will identify each stage within the process, determine what kind of hazard is present at that stage, and figure out how to prevent or reduce that hazard using predetermined controls. Finally, it’s time to create a detailed plan document outlining exactly what needs to be done – from pre-requisite programs, monitoring procedures, and corrective actions – while documenting all evidence along the way.
How can I maintain and audit my HACCP system?
After your HACCP system is established and documented, you need to make sure that it’s implemented and maintained properly. You should periodically conduct reviews of all pre-requisite programs, monitoring procedures, and corrective actions; take feedback from team members regarding the effectiveness of the system; and update documents as necessary. Additionally, carry out independent audits to evaluate performance and identify any additional improvements that can be made. Doing so will give you greater confidence that your team can deliver safe, quality food products every time.
What are the responsibilities of team members when implementing a HACCP plan?
Every member of the HACCP team has a role to play in the successful application and maintenance of the system. The HACCP Coordinator is typically responsible for ensuring that the HACCP plan is in compliance with regulatory requirements and successfully implemented by all team members. This individual also serves as a point of contact for any questions about HACCP and monitors the overall performance of the process. A Team Leader should implement the program, actively listen to any concerns from their colleagues, ensure compliance with regulations, investigate any possible food safety issues, and report back to the Coordinator on status updates. All team members must be trained thoroughly in how to perform their respective roles and must comply with every aspect of the established HACCP system.
The first step in implementing HACCP is to conduct a hazard analysis. This involves identifying potential hazards in your production process, including biological, chemical, and physical hazards. These hazards can include things like bacteria, allergens, foreign objects, and chemical contaminants.
Once you have identified these hazards, you will need to assess the risk of each one. This involves evaluating the likelihood and severity of each hazard and determining whether it is a significant risk to the safety of your products.
Once you have conducted a hazard analysis, the next step is to determine your critical control points (CCPs). CCPs are points in your production process where you can apply controls to prevent or eliminate a hazard. These controls can include things like temperature control, pH monitoring, and visual inspections.
To determine your CCPs, you will need to consider the hazards you identified in your hazard analysis, as well as the production processes that are critical to the safety of your products. You will then need to identify the points in these processes where you can apply controls to prevent or eliminate hazards.
Once you have identified your CCPs, the next step is to establish critical limits. Critical limits are the maximum or minimum values that must be met to ensure that a process is under control. These limits are based on factors like temperature, pH, and time.
To establish your critical limits, you will need to consider the hazards you identified in your hazard analysis and the controls you have in place at your CCPs. You will then need to set limits for each CCP that will ensure that the hazard is under control.
Once you have established your critical limits, the next step is to monitor your CCPs. Monitoring involves regularly measuring and recording the values of your critical limits to ensure that they are being met. This can be done manually or through automated systems.
If a CCP is found to be out of control, corrective actions must be taken to bring the process back under control. This can involve things like adjusting temperature or pH, re-inspecting products, or stopping production altogether.
Corrective actions are taken when a CCP is found to be out of control. These actions are designed to bring the process back under control and prevent the hazard from occurring. Corrective actions can include things like re-inspecting products, adjusting temperature or pH, or stopping production altogether.
It is important to have a clear plan in place for corrective actions, including who is responsible for taking them and how they will be documented and communicated.
Verification and validation are critical components of any HACCP system. Verification involves regularly checking that your HACCP plan is working as intended. This can involve things like reviewing records, conducting internal audits, and testing products for contaminants.
Validation involves ensuring that your HACCP plan is effective in controlling hazards. This can involve things like conducting challenge studies, where you intentionally introduce a hazard into your production process to see if your controls are effective.
Documentation and record-keeping are critical to the success of your HACCP system. You must have clear procedures in place for documenting and recording all aspects of your HACCP plan, including your hazard analysis, CCPs, critical limits, monitoring results, corrective actions, and verification and validation activities.
These records should be kept for a specified period and should be easily accessible for review by regulatory authorities or auditors.
Finally, it is important to be prepared for HACCP audits and inspections. Regulatory authorities may conduct inspections of your facility to ensure that you are complying with food safety regulations and operating your HACCP system effectively.
To prepare for these inspections, you should regularly review and update your HACCP plan, ensure that records are up to date-and accurate, and train your employees on HACCP procedures and protocols.
Implementing HACCP can be challenging, particularly for smaller businesses with limited resources. Some common challenges include:
– Lack of resources: Implementing HACCP can be time-consuming and require significant resources. To overcome this challenge, consider starting small and gradually expanding your HACCP system over time.
– Lack of expertise: HACCP can be complex, and it may be difficult to find employees with the necessary expertise to implement it. Consider providing training to your employees or hiring outside consultants to help you implement your HACCP system.
– Resistance to change: Implementing HACCP may require changes to your production processes, which can be met with resistance from employees. To overcome this challenge, involve your employees in the process and communicate the benefits of HACCP to them.
Conclusion
As a business owner, you are likely familiar with the term “regulatory compliance.” But what does that mean for your company? We explored seven principles of HACCP for regulatory compliance and showed you how to unlock the potential benefits that can come from implementing them in your business.
By learning about and understanding these principles, you can ensure that your company operates within applicable legal guidelines and avoids potential fines and other penalties. You can create a strong foundation for future growth and success with a little effort. If you want more clarification on this subject, you can find HACCP or PCP regulatory experts to help you!