
Medical Device Compliance in Canada: A Guide for Manufacturers
https://youtu.be/E0E0dohMpC4 So, you’re a medical device manufacturer eyeing the Canadian market? Great choice! But before you dive in, let’s talk
... Read more CAN Compliance
CAN Compliance
 USA Compliance
USA Compliance

https://youtu.be/E0E0dohMpC4 So, you’re a medical device manufacturer eyeing the Canadian market? Great choice! But before you dive in, let’s talk
... Read more
Introduction In the world of medical devices, ensuring patient safety and efficacy is paramount. The 510(k) submission process is a
... Read more
Navigating the complex medical device regulatory process can often feel like decoding a cryptic language. Manufacturers grapple with an intricate
... Read more
Are you a medical professional or someone involved in the healthcare industry? If so, you’ve likely come across the
... Read more
In this blog, we discuss the benefits, challenges, steps, and opportunities a medical device establishment license can offer manufacturers and
... Read more
The U.S. Food and Drug Administration (FDA) published two final guidelines on March 24, 2023, to help manufacturers of medical devices transition
... Read more
Medical devices play a crucial role in modern healthcare, providing patients with life-changing treatments and improving the quality of
... Read more
As a business owner in the medical industry, you understand the importance of providing quality products and services to your
... Read more
https://youtu.be/mtmJgcfh9gI If you are planning to, import, distribute, or sell medical devices in Canada or manufacture Class I medical
... Read more
As a medical device manufacturer, getting your product to market can be a complex process with various regulatory requirements to
... Read more
As technology continues to advance, the use of Software as Medical Devices (SaMD) is becoming more prevalent in the
... Read more
Introduction: Manufacturing and selling medical devices in Canada can be a complex process and requires adherence to Health Canada’s
... Read more
The price for an MDEL (Medical Device Establishment Licence) and MDL (Medical Device Licence) in Canada varies according to the
... Read more
What are Class 1 Medical Devices? Class 1 medical devices are the lowest risk category of medical devices, as
... Read more
Submitting a 510K Premarket Notification is an essential step in the process of getting medical devices approved by the
... Read more
The Medical Device License is a legal document required to manufacture, sell, and distribute medical devices. The Medical Device
... Read more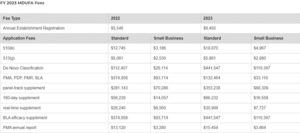
The FDA or U.S. Food and Drug Administration recently announced the Fiscal Year Medical Device User Fee (MDUFA) amendments. The
... Read more
The Medical Device License is a legal document that is required for the manufacture, sale, and distribution of medical
... Read more
Who Needs an MDSAP Certificate? Anyone looking to manufacture a Class II, III or IV medical device in Canada requires
... Read moreQuality Smart Solutions is a global consulting firm, engaged in providing strategic support for products including NHPs, pharmaceuticals, and medical devices, in areas such as Regulatory Support, Site Licensing, Clinical Development and more.