
FDA 510(k) pre-submissions vs PMA: What’s the difference?
When preparing to launch a medical device in the U.S., one of the first and most important decisions
... Read more
When preparing to launch a medical device in the U.S., one of the first and most important decisions
... Read more
Learn what MDL and MDEL mean, how they differ, and how to stay compliant with Health Canada regulations.
... Read more
Medical device shortage reporting requirements have recently changed, and it is essential for manufacturers and importers to stay informed.
... Read more
Learn how MDSAP helps meet Health Canada requirements and streamline audits for medical device compliance.
... Read more
Avoid FDA medical device submission errors and improve your approval chances with these expert tips.
... Read more
This article explores the differences between these designations and provides insights into how to obtain the necessary licenses for medical devices.
... Read more
Introduction In the ever-changing world of medical device manufacturing, ensuring your product complies with regulations is akin to navigating stormy
... Read more
As a business owner operating in dietary supplements and health products, you are undoubtedly aware of the ever-evolving regulatory
... Read more
In this blog, we discuss the benefits, challenges, steps, and opportunities a medical device establishment license can offer manufacturers and
... Read more
Medical devices play a crucial role in modern healthcare, providing patients with life-changing treatments and improving the quality of
... Read more
As a medical device manufacturer, getting your product to market can be a complex process with various regulatory requirements to
... Read more
https://youtu.be/mWaBQzsa1_M If you’re a business owner looking to expand your reach into the Canadian market, importing products may be a
... Read more
https://youtu.be/yvnTszySkgg If you’re in the medical device manufacturing business, you’re likely familiar with the FDA’s regulations, including FDA Furls. However,
... Read more
As a business owner, keeping up with regulatory requirements is a top priority. One such requirement is the FDA’s renewal
... Read more
Looking for a quality import service can be a daunting task, but with the right advice and tips, you can
... Read more
Introduction: Class 3 medical devices are subject to the highest level of scrutiny and require special attention when it comes
... Read more
Introduction: Medical devices are an important part of healthcare and play a critical role in the treatment of patients. Medical
... Read more
Navigating the medical device regulations set by Health Canada can be a complicated task, but knowing the specific classification
... Read more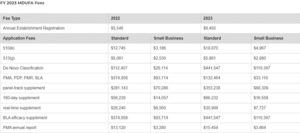
The FDA or U.S. Food and Drug Administration recently announced the Fiscal Year Medical Device User Fee (MDUFA) amendments. The
... Read more
In response to the COVID-19 public health emergency, FDA issued a declaration regarding the appropriateness of utilizing emergency use authorizations
... Read more
On December 11, 2021, Health Canada issued a notice regarding the annual adjustment of fees for drugs and medical devices
... Read more
Interim Order No. 3 Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19 (the third interim
... Read more
The advancement of technology is rapidly progressing, and the evolution can be observed all around us. As cutting-edge technology becomes
... Read more
It’s that time of year again! Any domestic and foreign establishments registered with US FDA must renew their registration annually
... Read more
As of March 1, 2021, Interim Order No. 2 replaces Interim Order No. 1 Respecting the importation and sale of
... Read more
Recently in Canada, the Minister of Health has signed an Interim Order to allow expedited access to COVID-19-related medical devices
... Read moreQuality Smart Solutions is a global consulting firm, engaged in providing strategic support for products including NHPs, pharmaceuticals, and medical devices, in areas such as Regulatory Support, Site Licensing, Clinical Development and more.
|
|
Thank you for Signing Up |
