As a business owner in the medical industry, you understand the importance of providing quality products and services to your customers. When it comes to medical devices, ensuring they are safe and effective is paramount. Working with a reputable medical device importer is critical for your business. In today’s global economy, there are countless options for medical device manufacturers and suppliers, but not all are created equal. Choosing the right partner can mean the difference between success and failure in your business.
In this article, we’ll explore why partnering with a reputable medical device importer is essential for your business and how it can help you achieve your goals. From ensuring compliance with regulations to providing high-quality products, we’ll cover everything you need to know to make informed decisions about your medical device supply chain. So, let’s dive in and discover why working with a reputable medical device importer is critical for your business.
What are the risks of working with unreliable medical device importers?
The medical industry is highly regulated and for a good reason. Medical devices are used to diagnose, treat, and prevent diseases and must be safe and effective. Unfortunately, not all medical device importers meet these standards. Working with an unreliable medical device importer can put your business at risk in several ways.
Firstly, an unreliable importer may provide low-quality products that do not meet regulatory requirements. These products may be more effective, safe, or harmful to patients. Secondly, an unreliable importer may not have the necessary documentation or certifications to prove the safety and efficacy of their products. This can result in delays or even failure to receive regulatory approval for your business. Finally, working with an unreliable importer can damage your reputation and credibility as a business. If your customers receive low-quality or unsafe products, they may lose trust in your ability to provide quality care.
What are the benefits of working with a reputable medical device importer?
Working with a reputable medical device importer provides several benefits for your business. Firstly, reputable importers have a proven track record of delivering high-quality products that meet regulatory requirements. They have the necessary certifications, documentation, and testing procedures to ensure their products are safe and effective. Knowing that your customers are receiving the best possible care gives you peace of mind.
Secondly, reputable importers have a deep understanding of the regulatory landscape. They stay up-to-date with regulation changes and have the necessary processes to ensure compliance. Avoiding regulatory issues can save your business time, money, and headaches.
Finally, working with a reputable medical device importer can enhance your reputation as a business. You can build trust and credibility with your customers by providing high-quality products. This can lead to increased business and better patient outcomes.
What qualities should I look for in a reputable medical device importer?
When choosing a medical device importer, there are several qualities to look for to ensure that you are working with a reputable partner. Firstly, look for importers with a proven track record of providing high-quality products that meet regulatory requirements. Check their certifications, documentation, and testing procedures to ensure that they have the necessary processes in place to ensure safety and efficacy.
Secondly, look for importers with a deep understanding of the regulatory landscape. They should stay up-to-date with regulatory changes and have the necessary processes to ensure compliance. Avoiding regulatory issues can save your business time, money, and headaches.
Finally, look for importers with a commitment to customer service. They should be responsive to your needs and provide clear communication throughout the importation process. This can help you build a strong working relationship and ensure that your business needs are being met.
What are the steps to importing medical devices safely and legally?
Importing medical devices safely and legally requires a thorough understanding of the regulatory landscape. The process can be complex and time-consuming, but working with a reputable medical device importer can simplify it.
The first step in importing medical devices is identifying the regulatory requirements for imported products. This includes understanding the necessary certifications, documentation, and testing procedures. Next, the importer must obtain the regulatory agencies’ required approvals and certifications. This can consist of FDA approval in the United States or CE marking in the European Union.
The importer can begin importing the products once the necessary approvals and certifications are obtained. This includes handling logistics such as shipping, customs clearance, and storage. Throughout the process, the importer must maintain documentation and records to ensure compliance with regulations.
Why is regulatory compliance substantial when importing medical devices?
Regulatory compliance is critical when importing medical devices. Medical devices are highly regulated to ensure they are safe and effective for patients. Failure to comply with regulatory requirements can result in delays or even failure to receive regulatory approval for your business.
Importers must stay up-to-date with regulatory changes and have the necessary processes to ensure compliance. This includes obtaining the required approvals and certifications from regulatory agencies, maintaining documentation and records, and handling logistics such as shipping, customs clearance, and storage.
Working with a reputable medical device importer can help ensure regulatory compliance.
Reputable importers have a deep understanding of the regulatory landscape and have the necessary processes in place to ensure compliance. Avoiding regulatory issues can save your business time, money, and headaches.
How can a reputable medical device importer help your business succeed?
Working with a reputable medical device importer can help your business succeed in several ways. Firstly, a reputable importer can provide high-quality products that meet regulatory requirements. Knowing that your customers are receiving the best possible care gives you peace of mind.
Secondly, a reputable importer can help you navigate the regulatory landscape. They stay up-to-date with regulatory changes and have the necessary processes to ensure compliance. Avoiding regulatory issues can save your business time, money, and headaches.
Finally, working with a reputable medical device importer can enhance your business’s reputation. You can build trust and credibility with your customers by providing high-quality products. This can lead to increased business and better patient outcomes.
What questions should I ask when choosing a medical device importer?
When choosing a medical device importer, several questions must be asked to ensure that you are working with a reputable partner. Firstly, ask about their track record of providing high-quality products that meet regulatory requirements. Check their certifications, documentation, and testing procedures to ensure that they have the necessary processes in place to ensure safety and efficacy.
Secondly, ask about their understanding of the regulatory landscape. They should stay up-to-date with regulatory changes and have the necessary processes to ensure compliance.
Finally, ask about their commitment to customer service. They should be responsive to your needs and provide clear communication throughout the importation process.
Conclusion
Choosing the right medical device importer is critical for your business. Working with a reputable partner can provide numerous benefits, including high-quality products, regulatory compliance, and enhanced reputation. When choosing a medical device importer, look for importers with a proven track record of providing high-quality products that meet regulatory requirements, a deep understanding of the regulatory landscape, and a commitment to customer service. By working with a reputable medical device importer, you can ensure that your business provides the best possible care to your customers.
Related Reading:
How Quality Smart Solutions can help
Our experts at Quality Smart Solutions are here to help and offer medical device importer-related related regulatory advice. We can also help support you in successfully securing your medical device license. We can help you by responding to potential information requests, keeping your license updated, and reviewing your device labels (510k Medical Device Registration, Facility Registration & FURLS, IVD Device Registration, and SaMD Classification.
Contact us today to learn more about how we can support your compliance needs during and after licensing! Please find our contact information:




 Are you running a business that deals with food, drugs, medical devices, cosmetics, or dietary supplements? If so, you must know the FDA establishment registration requirements to ensure your products meet legal standards and are safe for public consumption. The process of FDA establishment registration may seem daunting, but with the proper guidance, it can be a straightforward and manageable process.
Are you running a business that deals with food, drugs, medical devices, cosmetics, or dietary supplements? If so, you must know the FDA establishment registration requirements to ensure your products meet legal standards and are safe for public consumption. The process of FDA establishment registration may seem daunting, but with the proper guidance, it can be a straightforward and manageable process.







 Importing goods from other countries can be a lucrative business and a complex and tedious process. As an importer, you must navigate a maze of regulations, tariffs, and customs procedures that vary from country to country. That’s where an Importer of Record (IOR) comes in. An IOR is a professional service provider that can help you streamline your importation process, reduce risks, and save you time and money. In this article, we’ll explore the benefits of working with an IOR, including how they can help you navigate the legal complexities of importing goods, handle all the paperwork and documentation, and ensure that your goods are delivered to your doorstep on time and in good condition. Whether you’re a seasoned importer or just starting, this article will provide valuable insights on maximizing your importation process with the help of an IOR.
Importing goods from other countries can be a lucrative business and a complex and tedious process. As an importer, you must navigate a maze of regulations, tariffs, and customs procedures that vary from country to country. That’s where an Importer of Record (IOR) comes in. An IOR is a professional service provider that can help you streamline your importation process, reduce risks, and save you time and money. In this article, we’ll explore the benefits of working with an IOR, including how they can help you navigate the legal complexities of importing goods, handle all the paperwork and documentation, and ensure that your goods are delivered to your doorstep on time and in good condition. Whether you’re a seasoned importer or just starting, this article will provide valuable insights on maximizing your importation process with the help of an IOR.









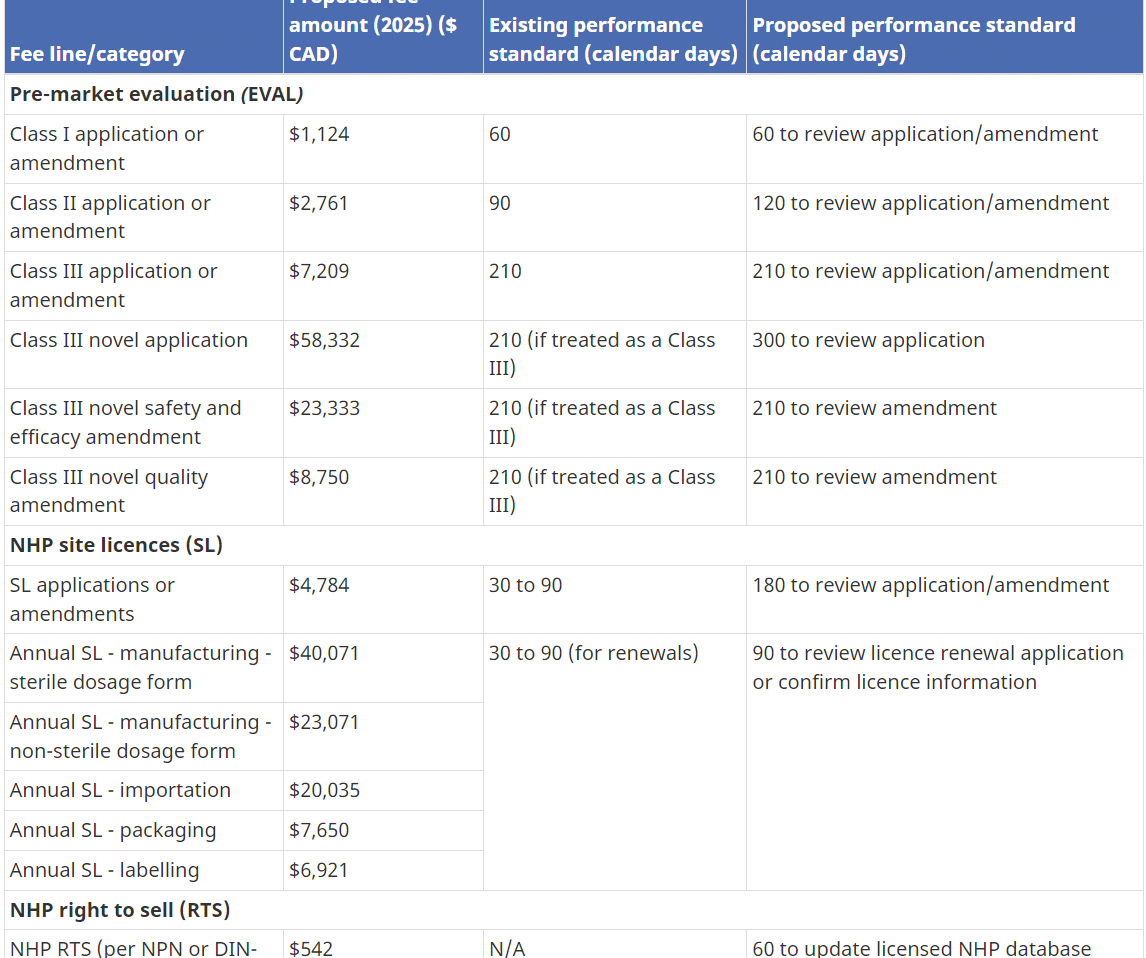
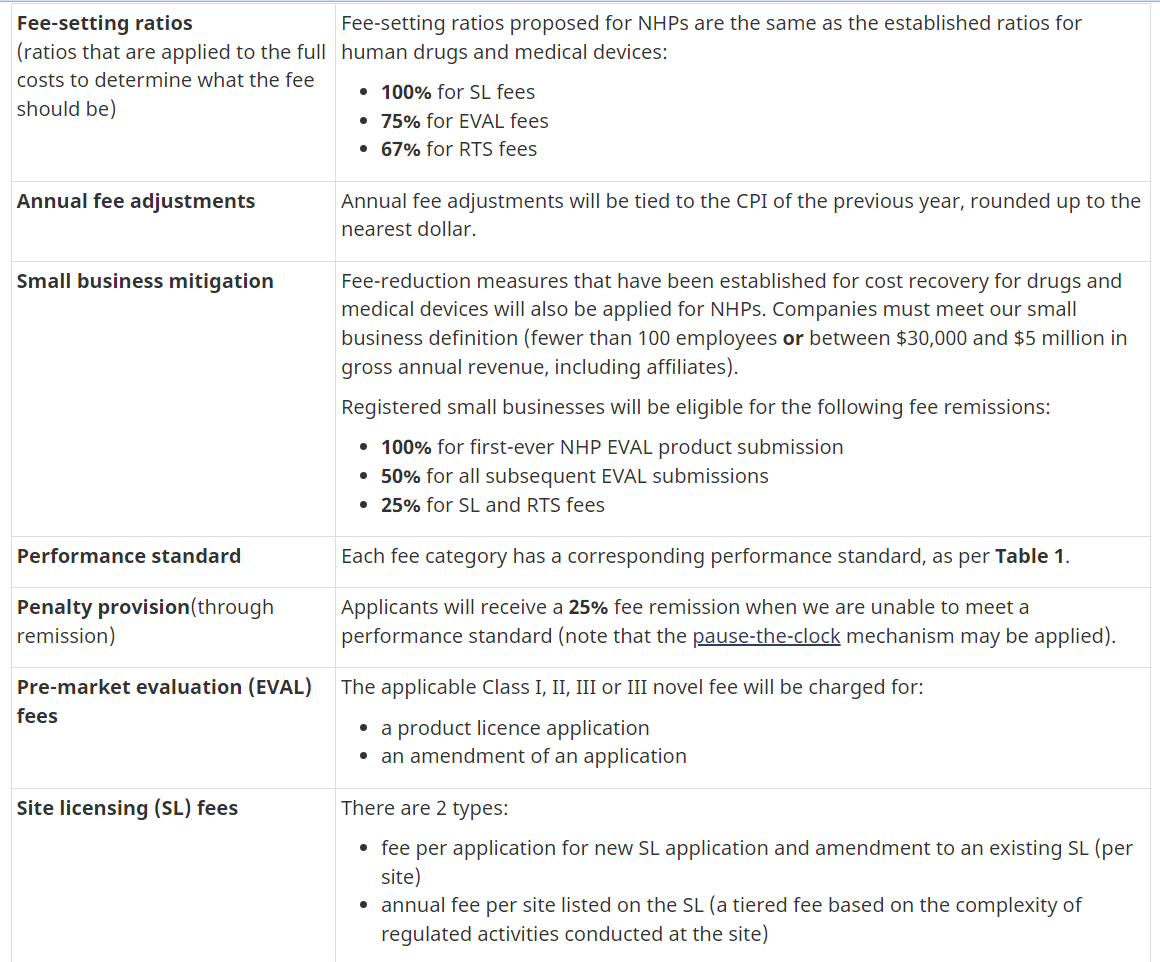
 Health Canada has the authority to set and charge health product fees under the
Health Canada has the authority to set and charge health product fees under the