
Navigating the process: your guide to obtaining a Natural Health Product License
Are you looking to bring your natural health product to market? Navigating the process of obtaining a license can be
... Read more CAN Compliance
CAN Compliance
 USA Compliance
USA Compliance

Are you looking to bring your natural health product to market? Navigating the process of obtaining a license can be
... Read more
Are you a business owner in the health and wellness industry? If so, you’re likely familiar with Health Canada’s Natural
... Read more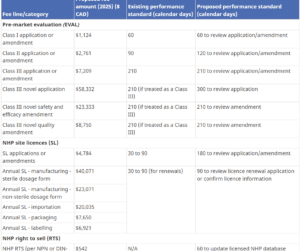
Health Canada has the authority to set and charge health product fees under the Food and Drugs Act (FDA). On
... Read more
What improvements are being made to support NHP site licensing? Internal template updates support: consistency between reviews-Fall 2023 Increased efficiencies
... Read more
This blog unpacks the distinctions between NHPs, Health Canada & FDA-approved drugs, and cosmetic product regulations that define these products.
... Read more
COVID had significantly impacted the processing of NHP product license applications with delays in the processing of all classes of
... Read more
Top 5 Tips to Successfully Secure an NPN Number In order to sell a Natural Health Product (NHP) in Canada
... Read more
Summary of Health Canada Licensing and Update Reports Product Licensing A combined 1390/5045 (28%) of NHP product license application submissions
... Read more
What to Expect After Submitting an NPN Application In order to sell a Natural Health Product (NHP) in Canada, you
... Read more
The “Sunshine” vitamin, Vitamin D, Vitamin D3 or Vitamin D2 as it is more commonly known. The term “sunshine” was
... Read more
In March 2020, Health Canada introduced interim measures to expedite the issuance of site licences for alcohol-based hand sanitizers, to
... Read more
Introduction: Identifying the correct application type has become increasingly crucial for Natural Health Product (NHP) applications. Incorrect classification can result
... Read moreQuality Smart Solutions is a global consulting firm, engaged in providing strategic support for products including NHPs, pharmaceuticals, and medical devices, in areas such as Regulatory Support, Site Licensing, Clinical Development and more.