What you should know about FDA Approvals for Drugs & Medical Devices
Introduction: FDA approvals can be a complex process, but understanding its important steps can give you insight into how drugs and medical devices enter the marketplace. From registering with the FDA to undergoing drug/device tests and inspections, find out all about FDA approvals here. What is the FDA and it’s role in regulating drugs &
... Read moreHow to comply with Health Canada Regulations for Medical Devices?
Introduction: Manufacturing and selling medical devices in Canada can be a complex process and requires adherence to Health Canada’s strict regulations. Understanding these regulations is essential for product safety, quality assurance, and compliance with the Canadian Medical Devices Regulations (CMDR). Understand Health Canada’s Safety Standards and Regulations: It’s important to ensure that a medical
... Read moreFDA Registration Renewals: FDA Increases Facility Enforcement

As a business owner, keeping up with regulatory requirements is a top priority. One such requirement is the FDA’s renewal registration. The process of registering your facility with the FDA can be confusing, but it’s essential to comply in order to avoid penalties or even shutting down your operation. Introduction: The American Food and Drug
... Read moreHealth Canada Medical Device License Renewals (MDL or MDEL) in 2023

As a Canadian medical device license (MDL) holder, you are subject to an annual license renewal procedure. The renewal process has two purposes, the first is to verify that the MDL will remain active and will continue to be sold in Canada. The second reason is to collect and analyze the appropriate information before invoicing
... Read moreWhat to Ask When Registering a Food Facility With the FDA

Quality Smart Solutions offers the best FDA Facility Renewal & Registration services in North America for Medical Devices, Food, and Drug FDA domestic and foreign facilities! The process of food facility registration can be complex, but knowing what is required and following the instructions carefully helps ensure a successful registration. In this guide, you will
... Read moreUnderstanding the Costs of MDL and MDEL Approvals

The price for an MDEL (Medical Device Establishment Licence) and MDL (Medical Device Licence) in Canada varies according to the categorization of the medical device. We will compare the prices for both MDELs and MDLs in this blog post according to their class. MDEL (Medical Device Establishment Licence) The total price of MDEL varies between
... Read moreFDA Fees for Medical Device Establishment Registration in 2023
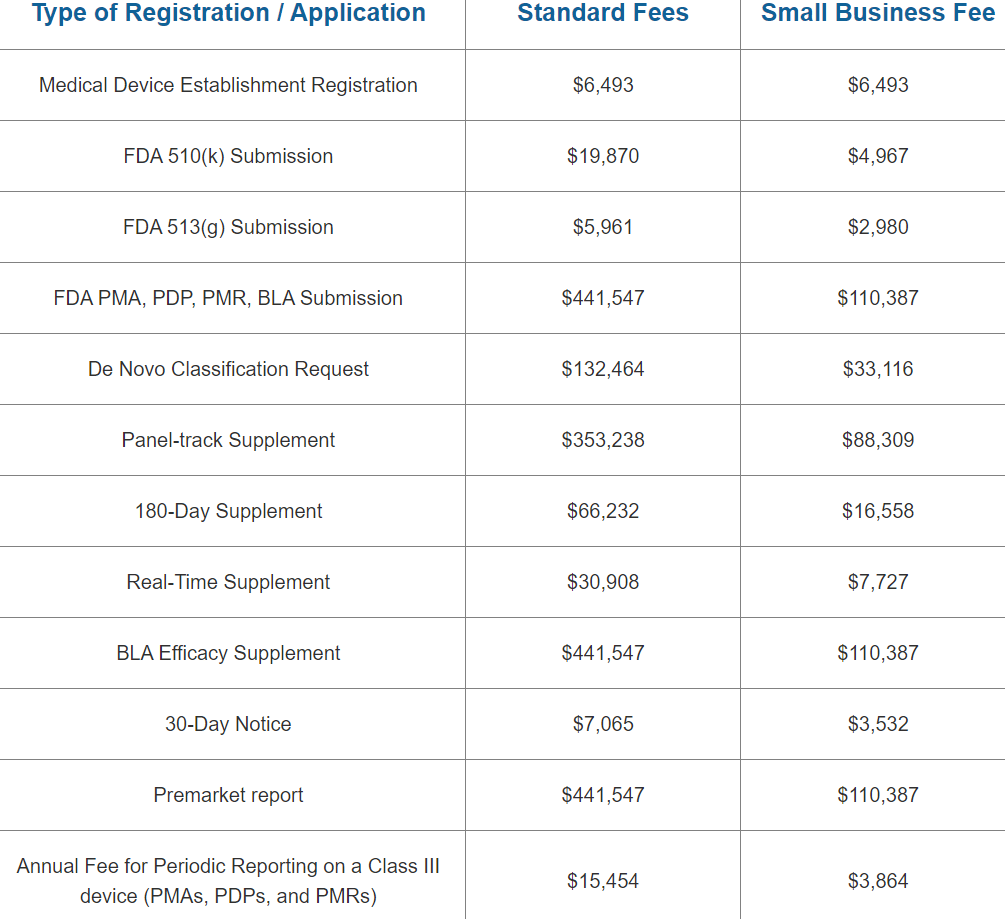
The cost to register an FDA Medical Device Establishment is USD $6,493 for 2023. The FDA’s fiscal year 2023 begins on October 1 and runs through September 30. Between October 1, 2022, and December 31, 2022, the yearly establishment registration fee must be paid. A reduced small company charge is NOT applicable to the
... Read moreWhat Companies Need to Understand About GRAS Ingredients

Introduction to GRAS: Understanding the regulations and safety risks associated with GRAS ingredients can be tricky, but it doesn’t have to be. This comprehensive guide gives you a detailed overview of GRAS ingredients and the regulations governing their use in food products, so you can get up to speed quickly. Your food scientists and our
... Read moreThe Importance of HACCP Consultants for Food Safety

Introduction: Food safety is an important part of any food business. It’s also a complicated one. There are many regulations and laws to take into consideration, and they can often be confusing to someone who isn’t trained in food safety or quality management. That’s where HACCP comes in: the Hazard Analysis and Critical Control Points
... Read moreEverything You Should Know About the NHP Database (LNHPD)

If you plan to manufacture, import, or sell a natural health product (NHP) in Canada, you need to understand the Licensed Natural Health Products Database (LNHPD). This official database lists every NHP that has been approved for sale with a Natural Product Number (NPN). Getting your product licensed and listed in the LNHPD is a
... Read moreHow to Choose the Right Importer: 5 Essential Tips

Looking for a quality import service can be a daunting task, but with the right advice and tips, you can find the perfect fit for your business. This decision becomes even more critical when importing CPG goods. Here are 5 essential factors to consider when choosing an import service provider. 1. Research Companies Thoroughly When
... Read moreUnderstanding Class I Medical Devices Approval With Health Canada

What are Class 1 Medical Devices? Class 1 medical devices are the lowest risk category of medical devices, as they are considered to have a low potential for harm to the user. These devices are typically simple in design and do not require a lot of regulatory oversight. Examples of class 1 medical devices include
... Read moreMDEL, DEL, DIN & PRMA Application Fee Amendments for 2023

PMRA Annual Fees reduced for Reporting and Applications: Health Canada is implementing a new approach beginning April 1, 2023. The PMRA will no longer contact registrants who fail to submit their volumetric sales report by the June 1st deadline. Registrants who do not submit their volumetric sales report by the June 1st deadline will face
... Read moreUnderstanding HACCP: 7 Principles Every Food Business Should Know
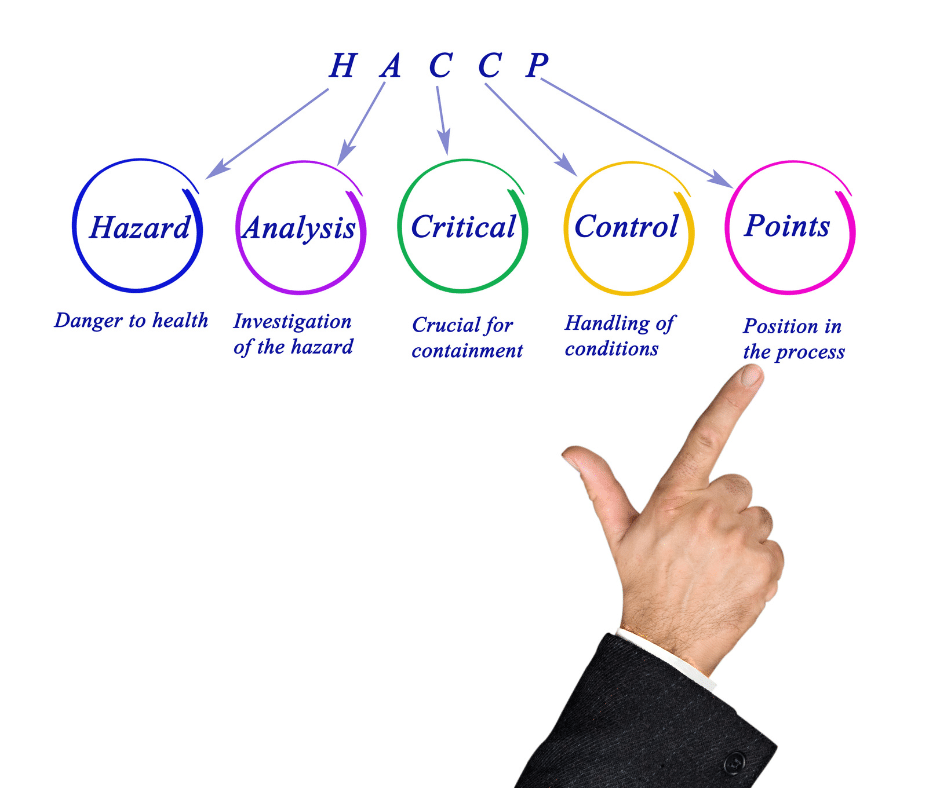
The purpose of a Hazard Analysis Critical Control Points (HACCP) plan is to identify and control any hazards that may arise during the process of manufacturing, storing, distributing, and consuming food products. Ensuring food safety is a critical component of the food industry. HACCP, or Hazard Analysis and Critical Control Points, is the system used
... Read moreMDR Amendments COVID-19 Medical Devices Importing & Sales
Background: Health Canada issued three interim orders to allow the importation and sale of medical devices used to diagnose, treat, mitigate, or prevent COVID-19. Interim orders were issued to speed up access to these medical devices in Canada during the pandemic. Interim Order No. 3 Concerning the Importation and Sale of Medical Devices for Use
... Read moreHealth Canada Labelling & Cosmetic Notification changes in 2023

Introduction: The sections relating to the disclosure of fragrance allergens because of the regulations go into effect two years following the date the Regulations concerning cosmetic ingredients. Six months following the date of registration, all other rules would take effect. In this article, we discuss the issues that lead to Health Canada’s cosmetic notification proposal
... Read moreGMP Certification Made Simple: What Companies Should Know

Are you considering obtaining GMP (Good Manufacturing Practice) certification for your business? The GMP certification process is crucial for ensuring your products meet rigorous safety, quality, and regulatory standards. Whether you are in the pharmaceutical, food, or dietary supplement industries, this certification not only helps maintain the highest quality but also supports compliance with global
... Read more