How a Trusted Importer of Record Supports Medical Device Compliance
As a business owner in the medical industry, you understand the importance of providing quality products and services to your customers. When it comes to medical devices, ensuring they are safe and effective is paramount. Working with a reputable medical device importer is critical for your business. In today’s global economy, there are countless options
... Read moreHow to Get a Medical Device Establishment Licence in Canada

Learn how to get your Medical Device Establishment Licence faster and stay compliant with Health Canada.
... Read moreHow Manufacturers Can Register With the FDA

Are you running a business that deals with food, drugs, medical devices, cosmetics, or dietary supplements? If so, you must know the FDA establishment registration requirements to ensure your products meet legal standards and are safe for public consumption. The process of FDA establishment registration may seem daunting, but with the proper guidance, it can
... Read moreThe Role of FSVP Certification in Global Food Safety

In today’s global food supply chain, ensuring the safety and quality of food products is critical to protecting public health and maintaining consumer confidence. With the increasing globalization of food production and distribution, there is a growing need for effective food safety management systems to identify and mitigate potential risks at every stage of the
... Read moreThe Role of Drug Identification Numbers in Prescription Compliance
The healthcare industry is constantly evolving, and one of the most significant advances has been implementing drug identification numbers (DINs). These unique numbers assigned to each medication are crucial in streamlining the prescription process and ensuring patient safety. With the rise of online pharmacies and the increasing complexity of drug interactions, DINs have become a
... Read moreStreamlining Imports With an Importer of Record

Importing goods from other countries can be a lucrative business and a complex and tedious process. As an importer, you must navigate a maze of regulations, tariffs, and customs procedures that vary from country to country. That’s where an Importer of Record (IOR) comes in. An IOR is a professional service provider that can help
... Read moreA complete guide to 510k submissions: Everything you should know
As a medical device manufacturer, getting your product to market can be a complex process with various regulatory requirements to fulfill. One of the most essential steps in getting your device approved for sale in the United States is submitting a 510(k) application to the FDA. However, navigating the 510(k)-submission process can take time and
... Read moreHealth Canada interim policy extension on importing and selling infant formula

Introduction: By extending the interim policy on the importation and sale of infant formulas, human milk fortifiers, and dietary products to treat inborn errors of metabolism, Health Canada intends to continue addressing shortages of infant formula and other foods for a particular nutritional purpose. This Health Canada notice also outlines the department’s plans for public consultation in
... Read moreEverything You Need to Know About Importing Products Into Canada

If you’re a business owner looking to expand your reach into the Canadian market, importing products may be a viable option. However, navigating the regulations and requirements for importing goods into Canada can take time and effort. From customs clearance to taxes and duties, there are many factors to consider before bringing your products across
... Read moreKey Strategies to Improve Drug Submission Success
In the pharmaceutical industry, proper drug submission management is crucial to the success of any drug development process. The submission process can be daunting, complex, and time-consuming. Still, it plays a vital role in ensuring that a drug gets approved by regulatory authorities and reaches critical patients. The consequences of poor submission management can be
... Read moreEmergency Alert: NNHPD proposed fees may lead to business closures
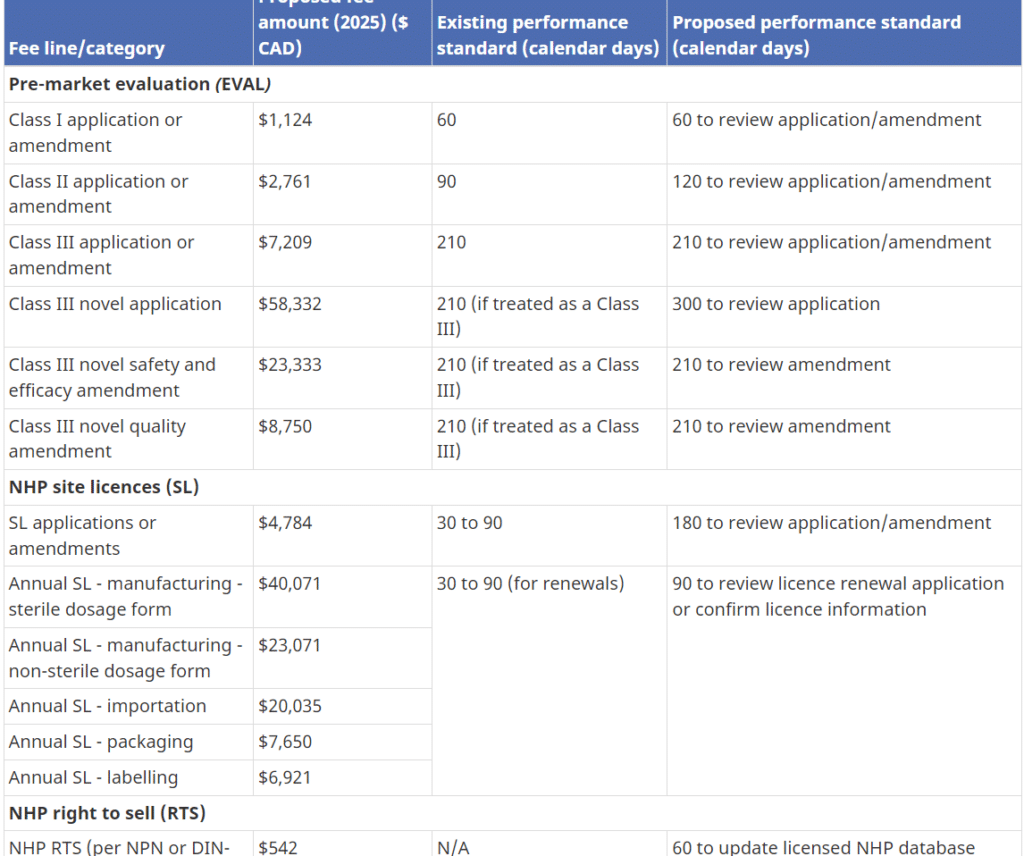
Health Canada has the authority to set and charge health product fees under the Food and Drugs Act (FDA). On May 12th, 2023, Health Canada published a proposal with a massive mandatory cost impact for the industry and consumers in the following areas: NHPs Annual Right to Sell (RTS) NHP Product License Applications (PLA) NHP
... Read moreFrom HACCP to CCPs: How Food Safety Standards Have Changed

The food industry has undergone a significant transformation in terms of safety and quality control over the years. From the early days of Hazard Analysis and Critical Control Points (HACCP) to the current Critical Control Points (CCPs) system, food safety standards have become more stringent and comprehensive. As a professional in the food industry, it’s
... Read moreNNHPD Improvements to support NHP site licensing
What improvements are being made to support NHP site licensing? Internal template updates support: consistency between reviews-Fall 2023 Increased efficiencies in Site Licensing-Summer/Fall 2022Continue risk-based administrative renewals. Looking to provide advance notice to lighten the burden of application and working with ROEB on leveraging inspection ratings Targeted updates to FPS User Guide*-December 15, 2022 -Test
... Read moreThe Importance of Food Safety Plans for Food Businesses

Are you considering getting your food safety certification? In today’s highly competitive food industry, ensuring your food products are safe for consumption is paramount. With the increasing number of foodborne illnesses and outbreaks, consumers are becoming more aware of the need for food safety certifications. As a food business owner, prioritizing food safety certifications not
... Read moreThe cost of HACCP Certification: Everything to know about the Investment

https://youtu.be/dVbug89UHBA HACCP (Hazard Analysis and Critical Control Points) certification is a food safety management system that helps businesses identify and control potential hazards in their food production process. While the cost of implementing a HACCP program may seem daunting, the benefits of certification can far outweigh the initial investment. In this guide, we’ll explore the
... Read moreWhy an FSVP Importer Is Essential for Food Safety

As our world becomes increasingly globalized, the importation of food has become a common practice. However, with growing concerns about food safety and compliance, we must ensure our food is safe and meets all regulatory requirements. This is where the FSVP importer comes in. FSVP, or the Foreign Supplier Verification Program, is a critical
... Read more