Choosing Between GRAS Notice and Self-Affirmed GRAS

If you’re introducing a new ingredient or additive into food products, understanding the GRAS Notice vs. Self-Affirmed GRAS pathways is essential. In the U.S., substances that are Generally Recognized as Safe (GRAS) can be used in food without requiring a formal FDA approval. But how do you determine which GRAS pathway is right for you?
... Read moreUnderstanding GRAS Substances and Their Role in Food Compliance

GRAS substances, or “Generally Recognized as Safe” substances, are essential to product development in the food, pharmaceutical, and cosmetic industries. If you’re in any of these fields, it’s important to understand how GRAS substances work so your products stay compliant, safe, and ready for market. Maybe you’re working on a new formulation or reviewing an
... Read moreEverything businesses should know about the Supplemented Foods Facts Table (SFFT)

Introducing the Supplemented Foods Facts Table (SFFT), your go-to guide for navigating the ever-changing world of supplemented foods. With consumer demand skyrocketing for these products, grasping the SFFT is vital for businesses looking to excel in this booming market. In this article, we’ll unravel everything you need to know about the SFFT, from its purpose
... Read moreHow Manufacturers Can Stay Compliant With Canadian Medical Device Rules

https://youtu.be/E0E0dohMpC4 So, you’re a medical device manufacturer eyeing the Canadian market? Great choice! But before you dive in, let’s talk about the regulatory hoops you’ll need to jump through. Selling medical devices in Canada comes with its own set of rules, and trust me, you want to be on the right side of these regulations.
... Read moreSelling Food in Canada: Navigating the Requirements for Conventional and Supplemented Products

https://youtu.be/QABD-2XIRz0 Canada is known for its diverse culinary landscape. If you’re considering selling food products in this country, you’re in for a rewarding venture. However, it’s essential to understand the specific requirements and regulations governing the sale of conventional and supplemented food items. This article will explore what it takes to sell food in Canada,
... Read moreA Guide to FDA 510(k) Clearance for Medical Devices
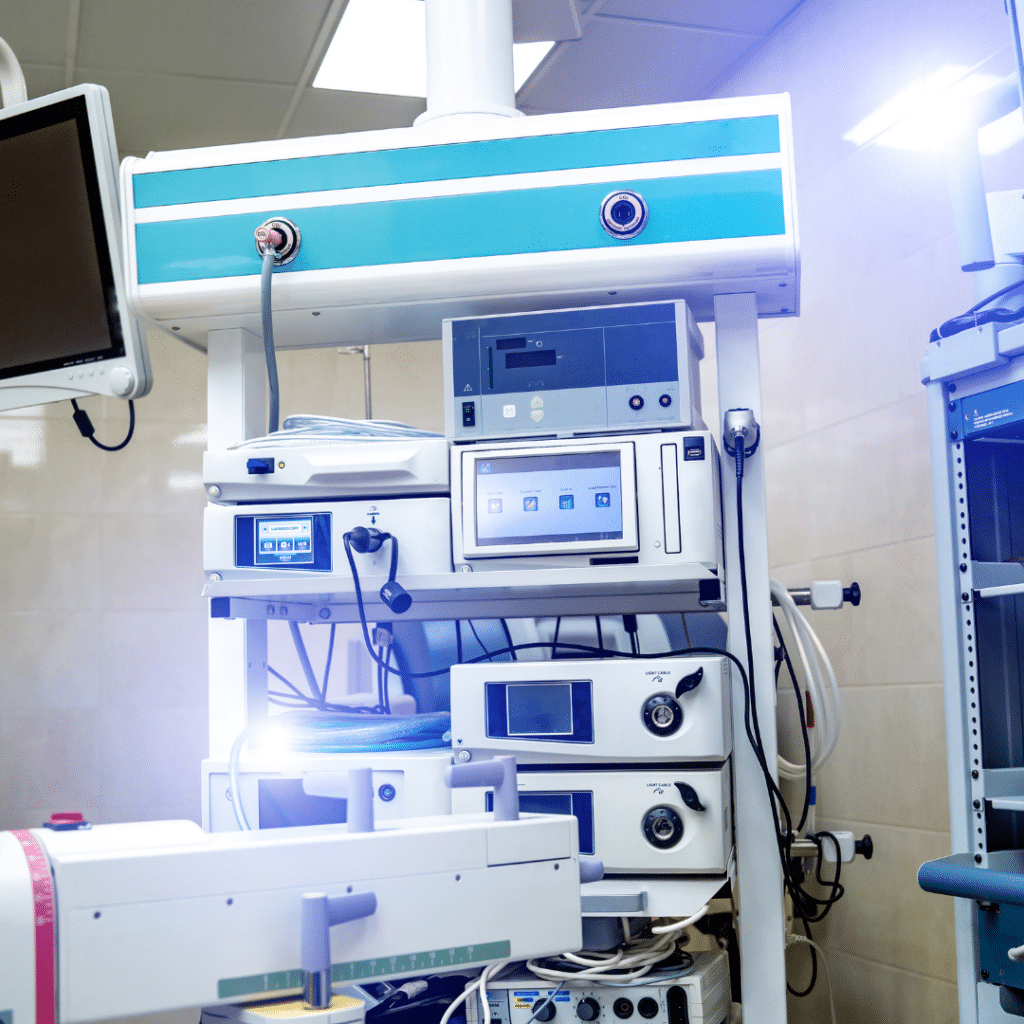
Navigating the intricate world of medical devices can be daunting, especially when it comes to ensuring their safety and effectiveness. In the United States, the FDA 510(k) clearance process is the critical checkpoint manufacturers must pass before introducing medical devices. This article will break down the regulatory complexities of FDA 510(k) clearance, including submission requirements,
... Read moreHow Drug Registration Works in the U.S. and What NDC Numbers Mean

Drug registration and NDC number might sound technical, but they play a big role in how medications make it to the U.S. market. If you’re in the pharmaceutical space, understanding how these systems work is key to getting your products approved, listed, and safely into the hands of patients. In this guide, we break it
... Read more