FDA Considerations Selling Foods in USA

Regulatory Considerations when Selling Foods and Beverages in the United States Selling food products in the United States requires compliance with various regulations to ensure consumer safety and public health. The regulatory framework is complex, involving multiple federal agencies, state laws, and industry standards. Here’s an overview of the key regulatory requirements: Federal Agencies:
... Read moreHealth Canada 2024 Changes for Nicotine Replacement Therapies (NRT)

Health Canada 2024 Changes for Nicotine Replacement Therapies (NRT) On August 22, 2024, the Honourable Mark Holland, Minister of Health, announced new measures aimed at preventing youth from being harmed by nicotine replacement therapies (NRTs). The following outlines the recent updates from Health Canada concerning NRTs. There is increasing concern that NRTs, particularly newer formats
... Read moreHow to Create Health Canada Compliant Food Labels

Designing a compliant food label is one of the most important steps when entering the Canadian market. The food labeling requirements in Canada are strict, detailed, and differ significantly from other countries such as the United States. For businesses exporting to Canada, understanding these rules is essential to avoid costly redesigns or product delays. Health
... Read moreNew Update on FDA Sodium Reduction Efforts
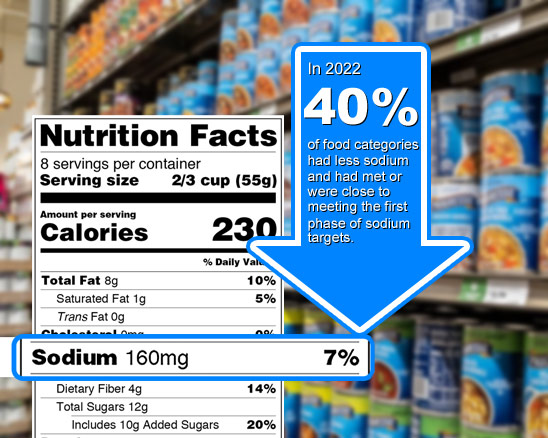
The FDA has taken a significant step forward in its ongoing sodium reduction initiative by issuing new, voluntary sodium reduction targets in a draft guidance, marking the launch of Phase II in their efforts. This follows Phase I, aimed at decreasing diet-related diseases associated with high sodium intake, where final voluntary sodium reduction goals were
... Read moreHealth Canada plans to share document about health products containing CBD this fall

This fall, Health Canada is anticipating sharing a policy consultation document about health products containing (CBD). This proposal has been in the works since 2019. In 2022, Health Canada released a report on health products containing cannabis. Released earlier this year, The Forward Regulatory Plan for 2024 to 2026 details regulatory initiatives that Health Canada
... Read more