What are the components of an NHP Label in 2023?
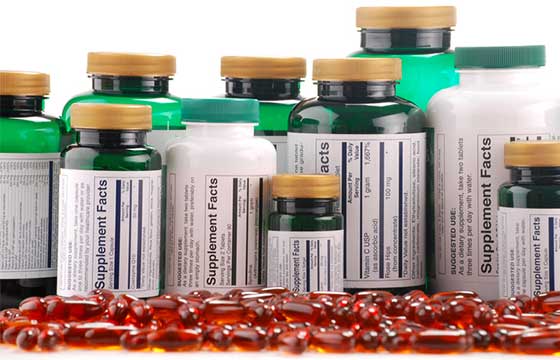
The product label is something we all look at in our daily lives, but not everyone is well equipped with the tools to read them efficiently. It can be challenging to try and navigate this space, especially for Natural Health Products (NHP), which can have multiple ingredients. First, you may be wondering what exactly is
... Read moreSupplemented Foods Proposed Regulatory Framework

Health Canada has been working on creating/revising the regulations with regards to Supplemented Foods for some time now. A recent Health Canada webinar indicated new proposals/consultation could be on the horizon. Health Canada anticipates publishing a new draft regarding the Supplemented Foods Regulations shortly (June 2021) in Canada Gazette Part I. Until now, supplemented foods
... Read morePublic Consultation on Medical Devices Clinical Trials Modernization Initiative

Health Canada has published a consultation paper on proposed changes to the regulation and oversight of clinical trials for drug, medical devices and natural health products (NHPs). The purpose of these changes is to improve the speed at which these trials are approved, to avoid stifling medical advances that could help improve the health of
... Read moreHow to sell a Dietary Supplement NHP on Amazon.ca (or online in Canada)

How to Sell Dietary Supplements or NHPs on Amazon or Other Online Platforms in Canada? What is an NHP? In Canada, a Natural Health Product (NHP) is regulated by Health Canada under the Natural Health Products Regulations. Under the regulations, it is defined as products containing naturally occurring substances including probiotics, herbal remedies, vitamins and
... Read moreCOVID-19 and Hard Surface Disinfectants

COVID-19 and Hard Surface Disinfectants – What You Need to Know In Canada, chemical products that are used to clean, sanitize or disinfect environmental surfaces and inanimate objects are regulated under different regulatory frameworks. As of recently, these products have been in high demand to help stop the spread of COVID-19. To provide some background,
... Read moreCancellation of MDELs for Non-Compliance with Annual Licence Review Requirements

Cancellation of Medical Device Establishment Licence for A Non-compliance With Annual Licence Review Requirements On May 27, 2021 Health Canada issued a bulletin about cancellation of Medical Device Establishment Licence (MDELs) for the sites that are not compliant with the annual licence review requirements. About 700 MDEL holders have not submitted an annual licence review
... Read more