
Understanding Class I Medical Devices Approval With Health Canada
What are Class 1 Medical Devices? Class 1 medical devices are the lowest risk category of medical devices, as they
... Read more
What are Class 1 Medical Devices? Class 1 medical devices are the lowest risk category of medical devices, as they
... Read more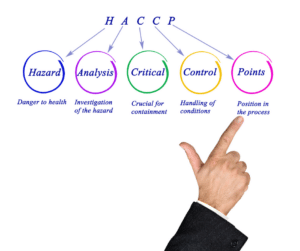
The purpose of a Hazard Analysis Critical Control Points (HACCP) plan is to identify and control any hazards that may
... Read more
Are you considering obtaining GMP (Good Manufacturing Practice) certification for your business? The GMP certification process is crucial for ensuring
... Read more
Introduction: Cosmetic labelling is an essential part of developing and marketing cosmetics. Companies must meet government requirements for labelling, including
... Read more
Introduction: A 510(k) clearance is a process by which medical device manufacturers notify the FDA of their intent to market
... Read more
Finding the right medical device regulatory consultant is one of the most important steps to ensure your product meets all
... Read more
If you want to know how to get your ingredient generally recognized as safe (GRAS) by the FDA, you are
... Read more
Introduction: On August 1, 2022, the FDA announced the final guidance on FDA’s policy regarding products labeled as dietary supplements
... Read more
Introduction: Class 3 medical devices are subject to the highest level of scrutiny and require special attention when it comes
... Read more
Introduction: FSMA stands for the “Food Safety Modernization Act” which is a set of laws enacted by the US government
... Read more
https://youtu.be/XRZ87ifRki0 Introduction: The Global Food Safety Initiative (GFSI) is an international organization that sets standards for food safety and quality
... Read more
Introduction: There are many reasons that you might want to get licensed as a food processor or retail operator. You
... Read more
Submitting a 510K Premarket Notification is an essential step in the process of getting medical devices approved by the
... Read more
Most regulations require you to follow HACCP principles rather than hold a formal certificate. HACCP certification demonstrates your commitment to
... Read more
If you’re in the food or ingredient industry, you’ve likely heard about the FDA GRAS database. It’s a vital resource
... Read more
Introduction: The FDA offers an online dietary supplement labeling guide that provides labeling requirements for supplement manufacturers. The guide covers
... Read more
https://youtu.be/XRZ87ifRki0 Ensuring the safety and quality of your food products is crucial for building trust with your customers and expanding
... Read more
Introduction Prior notice is a requirement for all food manufactured, processed, packed, or held outside the United States that is
... Read more
Introduction Food additives are chemicals added to food products to enhance flavor, texture, or color. Fortunately, there is a list
... Read more
Introduction: Meeting FSMA compliance requirements is one of the most important things a manufacturer can do to protect their business.
... Read more
Introduction Health Canada oversees the importation and sale of Veterinary health products (VHPs) in Canada through the VHP Notification Program.
... Read more
Introduction: Medical devices are an important part of healthcare and play a critical role in the treatment of patients. Medical
... Read more
Understanding the differences between GRAS and NDI is essential for any company developing food ingredients or dietary supplements in the
... Read more
HACCP, which stands for Hazard Analysis and Critical Control Points, is the cornerstone of food safety plans in the food
... Read more
Food is one of the most important parts of our lives. We eat it to stay alive, and we eat
... Read more
Introduction: FDA regulations for Class 1 medical devices can be daunting and confusing to navigate. However, with this easier-to-understand
... Read more
Navigating medical device regulations set by Health Canada can be challenging. However, knowing the correct classification of your medical device
... Read more
The Medical Device License is a legal document required to manufacture, sell, and distribute medical devices. The Medical Device Licence
... Read more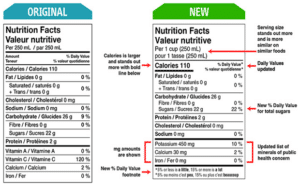
The Canadian Food Inspection Agency (CFIA) protects Canadians’ health and safety by ensuring food that reaches customers is safe, nutritious, and
... Read more
U.S.A RRA FDA Draft Guidance: In a recent U.S. Food and Drug Administration (FDA) draft guidance detailing its implementation
... Read more
Top Canada Cosmetic Regulations Cosmetic Notification Form (CNF) Canada is one of the most popular tourist destinations in the world,
... Read more
Background on FDA NAC Warning Letters: Navigating the FDA’s NAC system can be overwhelming. Understanding FDA warning letters is key
... Read more
Say you have a new ingredient and are ready to enter the US food market. To ensure this ingredient is
... Read more
The Canadian government has a set of regulations in place for natural health products (NHPs). These regulations help to ensure
... Read more
Foreign Supplier Verification Program (FSVP) Due to the increasingly global nature of today’s industry, an increasing number of businesses are
... Read more
The Medical Device License is a legal document that is required for the manufacture, sale, and distribution of medical
... Read more
Many drug industries are working in every country. To sell any OTC drug in Canada is not possible without
... Read more
The FSVP Plan is a requirement for many companies to be FSMA compliant. It is the responsibility of the company
... Read more
Nowadays almost all the facets of healthcare are well-equipped with the modern and latest technology tools, including software systems.
... Read more
Industry people and experts considering working with a company not registered with Health Canada should be aware of the regulatory
... Read more
https://youtu.be/XRZ87ifRki0 Since the publishing of the Safe Foods for Canadians Regulations in 2017, there is now a requirement for a
... Read more
If you’re selling natural health products in Canada, understanding NHPID and NPN is essential. These tools from Health Canada determine
... Read more
1. What is the NHP Licensing Process/what is needed for it? This process involves the submission of a product license
... Read more
In this blog, we’ll focus on answering your questions about the SFCR License and Preventive Control Plans, including why they
... Read more
Overview Manufacturers of prescription and non-prescription drugs are required to obtain a Drug Identification Number (DIN) before the drug product
... Read more
https://youtu.be/veCBMkYUWEU Market Analysis and Overview of CPG Market: Will CBD Products be considered an NHP? The consumer packaged goods (CPG)
... Read more
Do you want to learn about the food regulations for food sales in North America? This could include food facility
... Read more
Who needs a Foreign Site Reference Number (FSRN)? For Natural Health Products (NHPs) manufactured, packaged, and/or labeled by sites located
... Read more
As a license holder for Natural Health Products (NHPs), it is your responsibility to be aware of and educated on
... Read more
Learning how to classify your product correctly is one of the most important first steps when entering the Canadian market.
... Read more
Have you ever looked at a food label and wondered why all that information is there? Labels do more than
... Read more
What is a cosmetic? Under Section 2 of the Food and Drugs Act, a cosmetic is defined as “any substance
... Read more
Introduction: The market for animal and pet products is growing at a rapid pace. With that, there is also a
... Read more
What is HACCP? A common question that comes up in regulated industries, such as food manufacturing and processing, is “What
... Read more
1. What are over-the-counter drug products (OTC drugs)? Non-prescription drugs also called over-the-counter (OTC drugs), are health products that can
... Read more
Introduction Dietary supplements in the USA are very similar to natural health products (NHP regulations) in Canada and many products
... Read more
Filling out a Cosmetic Notification Form is a required step before selling any cosmetic product in Canada. Health Canada uses
... Read more
Top 5 Tips to Successfully Secure an NPN Number In order to sell a Natural Health Product (NHP) in Canada
... Read more
Natural Health Products have different characterization, identification, quantification, and purity standards that you should know about to ensure the quality
... Read more
Regulatory agencies across the globe, including the U.S. Food and Drug Administration (FDA), Health Canada, and the United Kingdom’s Medicines
... Read more
What is a claim? A claim is a representation for sale. It can be presented as “a word, a sentence,
... Read more
In general, all drug products (including over-the-counters and biologics) must be authorized for sale by Health Canada, before they can be
... Read more
All Natural Health Products (NHPs) sold in Canada are subject to the Natural Health Products Regulations (NHPR). Natural Health Products
... Read more
Introduction: The Consolidated Appropriations Act, recently passed by the US Congress, incorporates revisions to US cosmetics laws. The Modernization of
... Read more
Medicinal Mushrooms in powder, capsule or liquid form can be licensed as Natural Health Product (NHP) in Canada. To sell
... Read more
What is a Cosmetic? Cosmetics are a part of just about everyone’s life. Every day, most of us use products
... Read more
The classification of Foods and Natural Health Products (NHPs) can be considered difficult, grey, and convoluted. Sometimes even comes down
... Read more
The US Environmental Protection Agency (EPA) is saddled with the responsibility to register pesticides in the US. This process is
... Read more
What to Expect After Submitting an NPN Application In order to sell a Natural Health Product (NHP) in Canada, you
... Read more
Pet Foods and Human Food Labels are designed to look similar yet distinct. There are some similar label requirements and
... Read more
The advancement of technology is rapidly progressing, and the evolution can be observed all around us. As cutting-edge technology becomes
... Read more
How to Minimize the Risk of Border Detention Shipping Consumer Packaged Goods As an importer of consumer packaged goods, unanticipated
... Read more
Inspection of your food facility will help identify and fix any quality or processing deviations found regarding food safety. Review
... Read more
What is Hemp Seed Oil categorized as in Canada? In Canada, Hemp (Canna Sativa) seed oil can be categorized as
... Read more
Advertising is something almost all people experience and view daily. Yet, it can be challenging to navigate this space as
... Read more
Who Needs an MDSAP Certificate? Anyone looking to manufacture a Class II, III or IV medical device in Canada requires
... Read more
In the world of compliance and quality management, audits are a fact of life. Auditing verifies adherence to standards or
... Read more
Animal Supplements in the USA – What You Need to Know How are animal supplements regulated in the USA? Dietary
... Read more
The product label is something we all look at in our daily lives, but not everyone is well equipped with
... Read more
How to Sell Dietary Supplements or NHPs on Amazon or Other Online Platforms in Canada? What is an NHP? In
... Read more
COVID-19 and Hard Surface Disinfectants – What You Need to Know In Canada, chemical products that are used to clean,
... Read more
Natural Health Products (NHPs) are regulated by Health Canada under the authority of the Food and Drugs Act and the Natural
... Read more
Introduction The distinction between conventional and supplemented foods is something many food businesses find confusing. If you are developing or
... Read more
Introduction Have you ever wondered which Natural Health Product (NHP) application class your product belongs to? This is a question
... Read more
Health Canada recently published an additional notice regarding all interim orders relating to COVID-19 and Medical Devices. This notice is
... Read more
Earlier this summer, Health Canada provided a notice to industry on the regulatory considerations for non-medical masks or face coverings,
... Read more
What is a HACCP Plan? Food safety is always evolving and becoming more complex, as companies require constant updating with
... Read more
With the need for hand sanitizers significantly increasing for healthcare personnel and individuals during the current COVID-19 pandemic do help
... Read more
The Protecting Canadians from Unsafe Drugs Act (Vanessa’s Law) introduces amendments to the Food and Drugs Act that will improve Health Canada’s ability to
... Read more
All marketed drugs and health products have benefits and risks. All health products are carefully evaluated before they are licensed
... Read more
All drugs subject to the Food and Drug Regulations are required to gain premarket authorization prior to issuance of a
... Read more
When Health Canada authorizes a drug to be marketed in Canada, a Drug Identification Number (DIN) is issued to the
... Read more
The label and package are the first points of interaction between a health product and a consumer. Prior to the
... Read more
In continuation to the last article on Canadian legislations and requirements regarding the marketing of drugs and medical devices, this
... Read more
Health Canada is not only the national regulatory authority for issuing health product marketing authorizations, but it also oversees and
... Read more
Under the Food Safety Modernization Act (FSMA), the United States (U.S.) started graduated enforcement of the Foreign Supplier Verification Program
... Read more
Natural Health Product Brand Name Guidance Brand names are an important part of your marketing strategy, but as many of
... Read more
The Safe Food for Canadians Act (SFCA) and the Safe Food for Canadians Regulations (SFCR) are set to come into
... Read more
Unsure how your essential oils fit into Health Canada’s rules? The difference between a cosmetic and a Natural Health Product
... Read more
The European Food Safety Authority (EFSA) is a world renowned regulator which is known for its high scientific standards when
... Read more