
FDA Removes Key Barrier to Real-World Evidence for Drugs and Medical Devices
The U.S. Food and Drug Administration has announced a change to how it evaluates real-world evidence in regulatory reviews. In a
... Read more
The U.S. Food and Drug Administration has announced a change to how it evaluates real-world evidence in regulatory reviews. In a
... Read more
Preparing Instructions for Use (IFUs) for FDA Submission for Medical Devices Preparing Instructions for Use for FDA submission can feel
... Read more
When preparing to launch a medical device in the U.S., one of the first and most important decisions you’ll make
... Read more
If you’re planning to sell or distribute a medical device in Canada, you’re going to hear two terms over and
... Read more
Medical device shortage reporting requirements have recently changed, and it is essential for manufacturers and importers to stay informed. On
... Read more
If you’re working in the medical device space and looking to expand into the Canadian market, here’s a question for
... Read more
Submitting a medical device to the FDA can feel like navigating a maze. Even the most seasoned teams can overlook
... Read moreThe process of bringing medical devices to market in the United States involves stringent regulations imposed by the Food
... Read more
Introduction In the ever-changing world of medical device manufacturing, ensuring your product complies with regulations is akin to navigating stormy
... Read more
As a business owner operating in dietary supplements and health products, you are undoubtedly aware of the ever-evolving regulatory
... Read more
Introduction Health Canada MDALL is the key to legally marketing medical devices in Canada. If you are a manufacturer looking
... Read more
Introduction Navigating Health Canada’s licensing requirements can feel overwhelming, especially when your business depends on compliance to operate. Many companies struggle to
... Read more
Medical devices play a crucial role in modern healthcare, providing patients with life-changing treatments and improving the quality of
... Read more
Learn how to get your Medical Device Establishment Licence faster and stay compliant with Health Canada.
... Read more
As a medical device manufacturer, getting your product to market can be a complex process with various regulatory requirements to
... Read more
If you’re a business owner looking to expand your reach into the Canadian market, importing products may be a viable
... Read more
https://youtu.be/yvnTszySkgg If you’re in the medical device manufacturing business, you’re likely familiar with the FDA’s regulations, including FDA Furls. However,
... Read more
As a business owner, keeping up with regulatory requirements is a top priority. One such requirement is the FDA’s renewal
... Read more
Looking for a quality import service can be a daunting task, but with the right advice and tips, you can
... Read more
Finding the right medical device regulatory consultant is one of the most important steps to ensure your product meets all
... Read more
Introduction: Class 3 medical devices are subject to the highest level of scrutiny and require special attention when it comes
... Read more
Introduction: Medical devices are an important part of healthcare and play a critical role in the treatment of patients. Medical
... Read more
Navigating medical device regulations set by Health Canada can be challenging. However, knowing the correct classification of your medical device
... Read more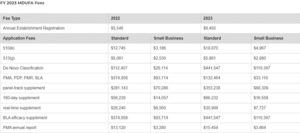
The FDA or U.S. Food and Drug Administration recently announced the Fiscal Year Medical Device User Fee (MDUFA) amendments. The
... Read more
In response to the COVID-19 public health emergency, FDA issued a declaration regarding the appropriateness of utilizing emergency use authorizations
... Read more
On December 11, 2021, Health Canada issued a notice regarding the annual adjustment of fees for drugs and medical devices
... Read more
Interim Order No. 3 Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19 (the third interim
... Read more
The advancement of technology is rapidly progressing, and the evolution can be observed all around us. As cutting-edge technology becomes
... Read more
It’s that time of year again! Any domestic and foreign establishments registered with US FDA must renew their registration annually
... Read more
As of March 1, 2021, Interim Order No. 2 replaces Interim Order No. 1 Respecting the importation and sale of
... Read more
Recently in Canada, the Minister of Health has signed an Interim Order to allow expedited access to COVID-19-related medical devices
... Read more