
Food or NHP: Distinguishing Between a Natural Health Product and a Supplemented Food
Supplemented foods and natural health products (NHPs) can sometimes pose challenges when trying to determine the correct regulatory pathway to
... Read more
Supplemented foods and natural health products (NHPs) can sometimes pose challenges when trying to determine the correct regulatory pathway to
... Read more
https://youtu.be/BEsWTYXGqAQ Summertime has finally arrived and with it comes hot temperatures and intense ultraviolet rays. Exposure to ultraviolet light is
... Read more
The Safe Food for Canadians Act (SFCA) and the Safe Food for Canadians Regulations (SFCR) are set to come into
... Read more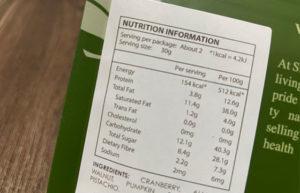
Health Canada implemented the Plain Language Labelling (PLL) initiative to help make the labels for non-prescription health products easier to
... Read more
In October 2014, the Natural and Non-prescription Health Products released a policy to manage the overall application review process. It
... Read more
Just a little background information first before I talk about the business aspect of capitalizing on your NPN. NPN stands
... Read more
On November 21, 2017 the Canadian Minister of Health announced the Proposed Approach to the Regulation of Canna. The public
... Read more
BACKGROUND Originally implemented in 1920 after the inception of the Federal department of Health in 1919, the Food and Drugs
... Read more
Some companies make the assumption that selling a dietary supplement product in Canada is no different than selling in the
... Read more
Health Canada recently announced their initiative to modernize the regulatory framework for health products and foods. This is required due
... Read more